Considerable advances in cancer therapies in recent decades have reshaped the prognosis of cancer patients. There are now estimated to be over 20 million cancer survivors in the USA and Europe, numbers unimaginable a few years ago. However, this increase in survival, along with the aging of the patient population, has been accompanied by a rise in adverse cardiovascular effects, particularly when there is a previous history of heart disease. The incidence of cardiotoxicity continues to grow, which can compromise the effectiveness of cancer therapy. Cardiotoxicity associated with conventional therapies, especially anthracyclines and radiation, is well known, and usually leads to left ventricular dysfunction. However, heart failure represents only a fraction of the cardiotoxicity associated with newer therapies, which have diverse cardiovascular effects. There are few guidelines for early detection, prevention and treatment of cardiotoxicity of cancer treatments, and no well-established tools for screening these patients. Echocardiography is the method of choice for assessment of patients before, during and after cancer treatment.
It therefore makes sense to adopt a multidisciplinary approach to these patients, involving cardiologists, oncologists and radiotherapists, collaborating in the development of new training modules, and performing clinical and translational research in a cardio-oncology program. Cardio-oncology is a new frontier in medicine and has emerged as a new medical subspecialty that concentrates knowledge, understanding, training and treatment of cardiovascular comorbidities, risks and complications in patients with cancer in a comprehensive approach to the patient rather than to the disease.
A taxa de sobrevivência dos doentes (dts) com cancro aumentou consideravelmente nas últimas décadas, havendo atualmente mais de 20 milhões de sobreviventes nos EUA e na Europa, números inimagináveis até há poucos anos. Para tal, muito contribuiu o aparecimento de novos fármacos (terapêuticas biológicas).
No entanto, estes benefícios na sobrevivência e o envelhecimento da população foram acompanhados de um aumento da taxa de efeitos adversos cardiovasculares, sobretudo se já havia doença cardíaca prévia. De facto, a incidência de cardiotoxicidade (CTX) tem sido continuamente mais evidente, comprometendo a eficácia das terapêuticas oncológicas (TO). São conhecidos os efeitos adversos cardíacos das TO tradicionais (antraciclinas e radioterapia torácica), como a insuficiência cardíaca. Contudo, esta representa apenas uma fração das manifestações de CTX, pois muitas das novas terapêuticas têm efeitos cardiovasculares diversos. As orientações clínicas existentes para fazer a deteção precoce, a prevenção e o tratamento da CTX dos tratamentos oncológicos, não abrangem todas as manifestações de CTX e ainda são poucas as ferramentas para a avaliação destes dts. A ecocardiografia é atualmente o método de escolha para avaliar os dts nas fases pré, durante e após a TO.
Dada a dimensão e relevância desta questão, faz todo o sentido falar de cardio-oncologia, uma nova subespecialidade médica. O número crescente de dts oncológicos com problemas cardíacos implica uma abordagem que deve ser partilhada entre cardiologistas, oncologistas e radioterapeutas.
Esta nova área do conhecimento médico deve também incluir uma componente formativa clínica, sendo também desejável a implementação de projetos de investigação clínicos e transacionais.
Cardiovascular disease and cancer together account for around 60% of deaths in the western world. In Portugal, data from the National Institute of Statistics for 2013 show that the leading cause of death is cardiovascular disease (29.5%), followed by cancer (24.3%).1
Nevertheless, survival rates for both diseases have increased in recent decades, as a result of significant advances in treatment. Five-year survival in the USA improved from 50% of patients diagnosed with cancer between 1975 and 1977 to 68% in those diagnosed between 1999 and 2005, and there are currently over 14 million cancer survivors, numbers unimaginable a few years ago.2,3 However, as survival improves, the late adverse cardiovascular effects of these therapies have become increasingly important.
It therefore makes sense to adopt a multidisciplinary approach to these patients, involving cardiologists, oncologists and radiotherapists in a cardio-oncology program. Interestingly, cardiovascular disease and cancer have risk factors in common, such as obesity and diabetes, and are often found in the same patient.
Cardiotoxicity is a common and well-known adverse effect of many conventional cancer therapies, especially anthracyclines and chest radiation, but may also occur with new biological therapies. It can affect survival and quality of life independently of cancer prognosis.
The most frequent adverse cardiovascular effects of cancer treatments include left ventricular dysfunction (symptomatic or asymptomatic), hypertension, arrhythmias, prolonged QT interval, thromboembolism and myocardial ischemia.4,5 Renal failure can also occur.
Unlike the cardiotoxicity associated with conventional cancer therapies (type I), that associated with biological therapies such as trastuzumab (type II) is usually reversible with discontinuation of treatment6,7 or treatable by medical therapy, such as hypertension associated with angiogenesis inhibitors such as sunitinib and bevacizumab.8,9 Furthermore, as therapeutic options evolve, conventional treatments are likely to be associated with one or more biological therapies, increasing the probability of cardiotoxicity.
Although cardiotoxicity associated with systemic cancer therapies is well understood, less is known about early and late cardiotoxicity due to new biological therapies or the early and late consequences of interactions between therapies.10 It is thus increasingly important to identify early biomarkers of cardiac involvement.11
The aim of this article is to provide a brief review of the state of the art and to recommend clinical practices that will improve the early detection and treatment of patients with cardiovascular complications arising from cancer therapies, by means of a multidisciplinary approach in a cardio-oncology program.
Anti-cancer drugsAnthracyclinesAnthracyclines (including doxorubicin, epirubicin, daunorubicin and idarubicin) are among the most commonly used drugs for chemotherapy and are especially effective in treating breast cancer and lymphoma. However, their effectiveness can be compromised by adverse cardiac effects, particularly heart failure (HF), which may appear early (weeks or months) or late (years) after treatment.
Recent research has shown that anthracyclines selectively inhibit the genetic expression of cardiac muscle. Doxorubicin binds to DNA by intercalating between specific bases and preventing the synthesis of DNA, RNA or both, thereby disrupting replication and transcription. The cardiac effects of the anthracyclines are associated with the inhibition of topoisomerase II and the formation of free oxygen radicals. These changes in genetic expression can lead to cardiomyocyte apoptosis and progressive loss of myofibrils in cardiac muscle.12,13
The main risk factor for the development of HF in anthracycline chemotherapy is the cumulative dose. The incidence of symptomatic cardiotoxicity with doxorubicin ranges between 5% for 400 mg/m2 to 48% for 700 mg/m2.14,15
It is estimated that more than half of patients treated with anthracyclines will develop cardiac changes within six years, and they have a five-fold greater probability of suffering HF than those not receiving these drugs.16
Other risk factors include older (>65 years) or younger (<18 years) age, female gender, hypertension, previous heart disease, diabetes, and previous chest radiation. Combined therapy with cyclophosphamide and taxanes, common in the treatment of breast cancer, can increase the risk of cardiotoxicity.17
TrastuzumabTrastuzumab, a monoclonal antibody with a high affinity for the HER2 receptor, has changed the natural history of patients with HER2-positive breast cancer, which accounts for 25% of breast cancers and is associated with a worse prognosis. Treatment with trastuzumab increases survival by 33% and reduces the risk of recurrence by 50% in these patients.18,19 However, it is associated with cardiotoxicity, with a greater incidence of symptomatic and asymptomatic left ventricular dysfunction (ranging from 4% as an adjuvant to 27% in metastatic disease).
The cardiotoxicity found with trastuzumab differs from that associated with anthracyclines, since it is dose-independent and does not lead to the ultrastructural alterations typical of the latter.20
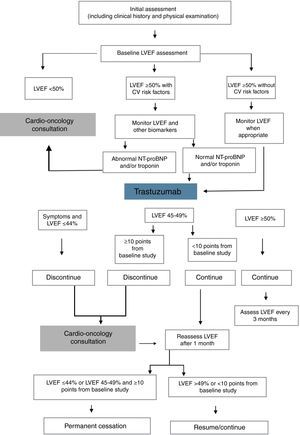
Proposed methods to reduce the cardiotoxicity of anthracyclines and trastuzumab include measurement of biomarkers such as troponin I and NT-proBNP, echocardiographic assessment of baseline left ventricular ejection fraction which is then monitored throughout treatment, and determination of ventricular global longitudinal strain (Figure 1). According to the results of these exams, cancer treatment may need to be suspended or discontinued and treatment for HF begun.21–24
Decision algorithm for detection and monitoring of types I and II cardiotoxicity during chemotherapy with trastuzumab. CV: cardiovascular; LVEF: left ventricular ejection fraction. Adapted from Rashi et al.60
This type of cardiotoxicity, characterized by left ventricular dysfunction, should be treated using the same drugs used for HF of other etiologies (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and beta-blockers), in accordance with the European and American guidelines for HF.25–27
Angiogenesis inhibitorsTumor growth is initially fed by nearby blood vessels, but when the tumor reaches a certain size, these vessels are no longer sufficient and in order to continue growing, the tumor must acquire the ability to generate new vessels, a process known as angiogenesis. To do so, tumor cells increase vascular pro-angiogenic factors such as endothelial growth factor (VEGF) and reduce angiogenesis-inhibiting factors.28–30
Hypertension is the adverse cardiovascular effect most frequently associated with VEGF inhibitors. The mechanisms behind VEGFI-induced hypertension are complex and multifactorial and are not fully understood. VEGF has vasodilatory effects by enhancing nitric oxide release; inhibition results in vasoconstriction, increased peripheral vascular resistance and hypertension. Other effects of VEGFI, such as endothelial dysfunction, vascular remodeling, arterial stiffness and capillary rarefaction, also appear to contribute significantly.32–34 A potent vasoconstrictor, endothelin-1 (ET-1), has also been implicated in VEGFI-induced hypertension. Its secretion appears to be increased in the presence of endothelial dysfunction, and there is evidence from clinical trials that raised ET-1 levels parallel increases in blood pressure (BP) in patients undergoing treatment with VEGFI.35
Virtually all patients treated with VEGFI have raised BP, and some develop hypertension; the greatest rise in BP is seen in the first cycle of VEGFI treatment. Hypertension is not an adverse effect of the treatment but a result of VEGFI-targeted treatment. This has raised the possibility that hypertension could be used as an indicator of the efficacy of the antiangiogenic response to VEGFI therapy and hence as a biomarker of good treatment outcome.30–32
As well as a dose-dependent rise in BP, angiogenesis inhibitors are also associated with increased risk of proteinuria. Some patients may develop glomerular disease or thrombotic glomerular microangiopathy leading to renal failure. These are reversible following immediate discontinuation of treatment.
There may be neurological complications in patients who develop hypertension, including posterior leukoencephalopathy syndrome, which is reversible by treatment discontinuation.
VEGFI-induced raised BP has a characteristic profile of rapid onset, within hours of beginning treatment, and systolic BP is more affected than diastolic. The incidence of hypertension is dose-related and is higher when multiple antiangiogenic agents are used in association.
The aim of optimal antihypertensive therapy is to enable VEGFI treatment to continue safely without altering the dose. To this end, a baseline cardiovascular assessment is recommended before therapy begins, including serial BP measurement. It is also important to assess renal function and proteinuria, since renal involvement can cause new-onset hypertension or worsen existing hypertension. The goal is not to exclude patients from such treatment but to assess their baseline risk and to monitor them closely. Antihypertensive therapy should aim to maintain BP below 140/90 mmHg, or 130/80 mmHg in the presence of diabetes or chronic renal failure (Table 1).33–36
| NCI-CTC | Recommendations | ESC | Recommendations |
|---|---|---|---|
| 0 | None | Optimal: <120/80 mmHg Normal: SBP 120-129 and/or DBP 80-84 mmHg | No intervention |
| 1 - Asymptomatic transient (<24 h) BP rise of >20 mmHg (diastolic) or >150/100 mmHg if BP previously normal | None | High normal: SBP 130-139 and/or DBP 85-89 mmHg | Lifestyle changes if >1 RF |
| 2 - Persistent recurrent rise of >20 mmHg (diastolic) or >150/100 mmHg if BP previously normal | Begin antihypertensive therapy (monotherapy) | Grade 1 HT: SBP 140-159 and/or DBP 90-99 mmHg | Lifestyle changes Then add BP drugs targeting <140/90 mmHg if TOD, CKD stage 3 or diabetes |
| 3 - BP >160/100 mmHg | More aggressive antihypertensive therapy | Grade 2 HT: SBP 160-179 and/or DBP 100/109 mmHg | Lifestyle changes Immediate BP drugs targeting <140/90 mmHg if >1 RF |
| 4 - Malignant HT, transient or permanent, neurological deficit, or hypertensive crisis | Urgent intervention | Grade 3 HT: ≥180/110 | Lifestyle changes Immediate BP drugs targeting <140/90 mmHg if symptomatic CVD, CKD stage ≥4 or diabetes with TOD |
BP: blood pressure; CKD: chronic kidney disease; CVD: cardiovascular disease; DBP: diastolic blood pressure; ESC: European Society of Cardiology guidelines (2013); HT: hypertension; NCI-CTC: National Cancer Institute Common Terminology Criteria for Adverse Events v. 4.0 (2010); RF: risk factor; SBP: systolic blood pressure; TOD: target organ damage.
Various antihypertensive drug classes are used to treat hypertension in cancer patients. All are effective, and no class has been shown to be superior to any other, but care must be taken with the non-dihydropyridine calcium channel blockers verapamil and diltiazem, which are also CYP3A4 inhibitors. Since endothelial nitric oxide is a putative mediator of angiogenesis, agents such as nitrates and nebivolol that increase nitric oxide levels are recommended for hypertension treatment in these patients.33
When therapy begins, regular BP assessment is recommended during the first treatment cycle and every 2-3 weeks thereafter; patients should be advised to measure their own BP at home. Assessment should be more frequent if patients are also taking drugs that increase the risk of hypertension such as anti-inflammatory agents or erythropoietin.
If systolic BP reaches ≥200 mmHg or diastolic BP reaches ≥100 mmHg, the dose of anticancer drugs should be reduced or treatment suspended. The dosage should be maintained at the highest level that the patient can tolerate, aiming to reduce the short-term risk of events associated with hypertension (stroke, myocardial infarction, or HF) while maintaining effective doses of antiangiogenic drugs.
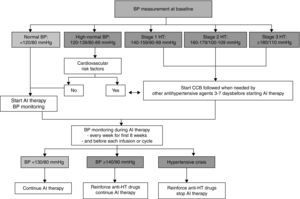
There are no specific guidelines for VEGFI-induced hypertension, and so therapy should be based on the European Society of Cardiology (ESC) and National Cancer Institute guidelines37,38 (Figure 2).
Initial assessment and monitoring of hypertension during therapy with angiogenesis inhibitors. AI: angiogenesis inhibitor; BP: blood pressure; CCB: calcium channel blocker; HT: hypertension. Adapted from Ederhy et al.36
Radiation of the heart can lead to radiation-induced heart disease (RIHD), which is related to cumulative dose (the product of the number of treatments and radiation dose) and can be exacerbated by chemotherapy, especially anthracyclines. Manifestations of RIHD can be acute but are usually only seen years after treatment. It has various adverse effects, including HF, coronary disease, pericarditis, valve disease and arrhythmias. It may also be aggravated by risk factors that are common to heart disease and cancer, such as obesity, sedentary lifestyles, diabetes, hypertension and smoking.
The dose-dependent increase in cardiovascular disease following chest radiation is well documented, especially with lymphoma and breast cancer (particularly of the left breast), and cardiovascular disease is the leading non-cancer cause of death in these patients. Studies analyzing the long-term risk/benefit ratio have shown that the positive effect of radiotherapy may in fact be partly canceled out by cardiac complications.39,40 However, these data are mostly retrospective and based on treatment protocols that are no longer used. The prevalence of RIHD is unknown with new radiotherapy protocols, which include planning the area to radiate by three-dimensional computed tomography (CT), using lower doses and reducing the size of the radiation field, in order to protect the heart. In breast cancer, for example, CT planning avoids including the heart in the radiation field, while excluding the internal mammary lymph nodes enables doses to the heart to be reduced. Another technique is fractionating the radiation dose; some studies have shown that the more the dose is fractionated, the lower the incidence of acute pericarditis and myocardial necrosis. These new protocols are intended to reduce the incidence of RIHD, but there are as yet no long-term follow-up data.
Ionizing radiation can damage virtually all cardiac structures. Its effects on the vascular system are seen at both microvascular and macrovascular levels. At the microvascular level, it leads to loss of endothelial cells, triggering an inflammatory response, vascular injury and ischemia. The resulting fibrosis appears to be due to the process of cell repair rather than to the direct effect of radiation. Macrovascular changes include accelerated arteriosclerosis and coronary artery obstruction, leading to acute coronary syndromes at younger ages.
Diffuse fibrosis after radiotherapy, which can be identified histologically in both myocardium and pericardium, can result in restrictive myocarditis and constrictive pericarditis.41–45
There are no guidelines for cardiac monitoring in these patients. To minimize the risk of RIHD, patients at high risk for cardiac events should be identified before beginning radiotherapy and, when appropriate, cardiac assessment should be repeated for the rest of their lives.
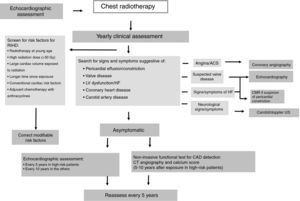
In patients who have undergone chest radiotherapy, cardiac function should be reassessed every 10 years, or every five years in those considered at high risk for RIHD (Figure 3).46
Algorithm for patient management after chest radiotherapy. ACS: acute coronary syndrome; CAD: coronary artery disease; CMR: cardiac magnetic resonance; CT: computed tomography; HF: heart failure; LV: left ventricular; RIHD: radiation-induced heart disease; US: ultrasound. High-risk patients defined as having had anterior or left-side chest irradiation with ≥1 risk factors for RIHD. Adapted from Lancellotti et al.46
A cardio-oncology program should have three main components: (1) cardio-oncology clinic; (2) training; and (3) research.
Cardio-oncology clinicObjectives- -
to provide specialized cardiological care to patients with cancer and a history of cardiovascular disease or who develop cardiac complications during cancer therapy;
- -
to optimize cardiac care in cancer patients undergoing potentially cardiotoxic therapy;
- -
to improve knowledge of cardiac complications of cancer treatments;
- -
to promote early detection of cardiotoxicity (using clinical, laboratory and imaging biomarkers, of which echocardiography is the most important) and to establish intervention strategies to optimize cardiological care;
- -
to improve patients’ prognosis through a multidisciplinary and integrated approach involving different health professionals (physicians, nurses and technicians).
The clinic's protocols for monitoring cardiotoxicity should include three steps (Table 2): assessment before beginning cancer therapy, particularly in patients with cardiovascular risk factors; assessment during treatment, in order to detect and treat cardiovascular complications promptly; and monitoring after treatment (Tables 3 and 4). The use of a risk score enables better identification of patients who should be referred for cardio-oncology consultation (Table 5).
Steps in the monitoring of cardiac events during cancer therapy.
| Before therapy | - Risk assessment - Identification and control of cardiovascular risk factors - Personalization of cancer therapy to minimize cardiovascular risk |
| During therapy | - Prompt detection of cardiotoxicity - Identification and control of cardiovascular risk factors |
| Following therapy | - Monitoring of symptoms and scheduled cardiovascular assessment - Control of cardiovascular risk factors |
Cardiotoxicity risk score based on cancer drugs used and patient-related risk factors.
| Medication-related risk | Patient-related risk factors |
|---|---|
| High (risk score 4): Anthracyclines; cyclophosphamide; trastuzumab | - Age <15 or >65 years - Female gender - Hypertension, diabetes - Cardiomyopathy, HF, CAD, PAD - Prior anthracycline chemotherapy |
| Intermediate (risk score 2): Docetaxel; pertuzumab; sunitinib; sorafenib | |
| Low (risk score 1): Bevacizumab; dasatinib; imatinib; lapatinib | |
| Rare (risk score 0): Etoposide; rituxumab; thalidomide |
CAD: coronary artery disease; HF: heart failure; PAD: peripheral arterial disease.
Risk categories by drug-related risk score plus number of patient-related risk factors: risk score >6: very high, 5-6: high, 3-4: intermediate; 1-2: low, 0: very low.
Recommendations for monitoring of cardiac events during cancer therapy.
| Risk of cardiotoxicity | Patients | Monitoring |
|---|---|---|
| High | Structural heart disease or very low LVEF | - Follow-up in cardio-oncology consultations - Potentially cardiotoxic therapy only in exceptional circumstances |
| Intermediate | Cardiovascular risk factors and LVEF >40% | Baseline assessment: - ECG; blood tests (creatinine, HbA1c, lipids) - Troponin, NT-proBNP - Echocardiogram (LVEF and strain) Assessment during treatment: - Troponin in each cycle - Echocardiogram (LVEF and strain) - Consider cardiovascular therapy (beta-blockers, ACE inhibitors, statins) Long-term assessment: - ECG plus echocardiogram (strain) plus troponin and NT-proBNP at the end of treatment, after 6 and 12 months, and then every 3-4 years |
| Low | Asymptomatic, no cardiovascular risk factors or structural heart disease | Baseline assessment: - ECG; blood tests (creatinine, HbA1c, lipids) - Troponin, NT-proBNP - Echocardiogram (LVEF and strain) Assessment during follow-up: - ECG plus echocardiogram (LVEF and strain) at end of treatment |
ACE: angiotensin-converting enzyme; ECG: electrocardiogram; LVEF: left ventricular ejection fraction.
Adapted from Fernandez et al.50
Criteria for referral for cardio-oncology consultation.
| Patients at high or intermediate risk for optimization of therapy (ACE inhibitors, beta-blockers, statins) - Prior treatment with doxorubicin ≥300 mg/m2 and/or mediastinal radiotherapy ≥30 Gy - Structural heart disease, HF, coronary disease or arrhythmias - Uncontrolled hypertension, dyslipidemia or diabetes |
| Alterations on ECG or baseline echocardiogram or during follow-up: - Fall in LVEF of >10% with baseline LVEF ≥55% - Abnormal GLS (>-19%) or >15% fall - Positive troponin - Chest pain, dyspnea, syncope, arrhythmias - Hypertension refractory to therapy |
ACE: angiotensin-converting enzyme; ECG: electrocardiogram; GLS: global longitudinal strain; HF: heart failure.
Appropriate algorithms that are easy to apply in clinical practice are needed in order to enable prompt detection and monitoring of cardiotoxicity.47–50
Finally, continuous quality control should be implemented, possibly using the ‘plan, do, check, act’ (PDCA) method. The results will permit analysis of the quality of the program, evaluating therapeutic decisions, outcomes, safety profile, and patient satisfaction measured by questionnaires. There should also be recommendations on how to combat risk factors, including patient education. Records need to be kept and study and training plans, both undergraduate and postgraduate, should be prepared.55
TrainingAs cardio-oncology is a new frontier in medicine, it should also include a training component, both undergraduate and postgraduate, as recommended by the ESC. It is important that there should be a period of training in cardio-oncology for both oncologists and cardiologists, at a basic or advanced level according to individual options.51–58
ResearchClinical and translational research projects should be organized aimed at early identification of cardiotoxicity and of individual susceptibility to developing adverse cardiac effects.
Cardiotoxicity is an increasing concern in clinical and preclinical trials of new drugs. Adverse cardiac effects often result in discontinuation of cancer therapy. There is therefore a growing need for better prediction of the risk of cardiotoxicity of new drugs at an early stage in their investigation.59–61
ConclusionSurvival rates of cancer patients have increased, due to new therapies. However, this notable achievement may be overshadowed by the adverse effects of these therapies on the cardiovascular system. Manifestations of cardiotoxicity may be acute or late, months or years after the end of treatment. It can also exacerbate or unmask existing cardiac conditions.
The development of cardiovascular disease during cancer therapy may lead to changes in the therapeutic regime, such as alterations in dosage, duration of cycles, and temporary or permanent discontinuation of therapy, and can thus reduce its effectiveness.
The new medical subspecialty of cardio-oncology has arisen in response to the need to detect cardiovascular involvement as early as possible and to optimize cardiological treatment in cancer patients, both during therapy and long-term. The importance of a multidisciplinary approach is recognized by European and American medical societies and other organizations, and should become standard in the follow-up of these patients. Close collaboration between specialties will also help establish clinical guidelines and clinical and translational research protocols that can respond to the need to predict, prevent and treat cardiotoxicity.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fiuza M, Ribeiro L, Magalhães A, Sousa AR, Menezes MN, Jorge M, et al. Organização e implementação de uma consulta de cardio-oncologia. Rev Port Cardiol. 2016;35:485–494.