Congenital long QT syndrome (LQTS) can present as syncope or seizures, secondary to polymorphic ventricular tachycardia, mimicking a primary seizure disorder.
In patients treated with an implantable cardioverter-defibrillator (ICD), the recurrence of arrhythmias with subsequent frequent therapeutic shocks may cause adverse reactions, which can be psychogenic.
We report the case of a 22-year-old woman with syncope and seizures who was diagnosed in childhood as epileptic and in whom LQTS was diagnosed only in adulthood.
Beta-blocker therapy failed and an ICD was implanted. However, as arrhythmias persisted, left cardiac sympathetic denervation was performed. After surgery, three-month follow-up showed a significant reduction in arrhythmias.
The genetic study identified a heterozygous mutation, c.1817 C>T p.S606F, on the KCNH2 gene that has not previously been reported in the literature.
We also report the rare occurrence of an electrical storm in the course of H1N1 infection.
This case illustrates the difficulties in the diagnosis and treatment of LQTS. The possibility of a common genetic basis for arrhythmic diseases and epilepsy is discussed.
A síndroma de QT longo congénita (SQTL) pode manifestar-se por síncopes ou convulsões recorrentes, no contexto de taquicardia ventricular polimórfica, podendo simular epilepsia.
Nos doentes tratados com cardioversor-desfibrilhador implantável (CDI) a recorrência de arritmias com consequente terapêutica com choques frequentes pode conduzir a reacções adversas, nomeadamente psicogénicas.
Apresentamos o caso de uma doente de 22 anos com síncopes e crises convulsivas, cujo diagnóstico era desde a infância de epilepsia, e em quem a SQTL foi diagnosticada apenas em idade adulta. Por falência da terapêutica beta-bloqueante implantou CDI, e por persistência de arritmias foi submetida a simpaticectomia cardíaca esquerda. O follow-up pós-cirurgia aos 3 meses mostrou redução significativa do número de arritmias.
O estudo genético identificou uma mutação patogénica no gene KCNH2 (SQTL tipo 2), em heterozigotia, a mutação c.1817C >T p.S606F, ainda não descrita na literatura. Relatamos também a rara ocorrência de tempestade arrítmica no contexto de infecção a H1N1.
O caso clínico ilustra as dificuldades quer do diagnóstico quer do tratamento da SQTL. É discutida a possibilidade duma base genética partilhada entre a doença disrítmica e neurológica.
Congenital long QT syndrome (LQTS) is an inherited arrhythmogenic disorder characterized by prolongation of the QT interval, with high risk for severe ventricular arrhythmias, especially polymorphic ventricular tachycardia (torsade de pointes). It is frequently manifested by syncope and sudden death (SD).1 LQTS is a channelopathy caused by mutations in the genes that code for proteins in ion channels of the cardiac cell membrane.1 Twelve genes linked to the condition have been identified, and there is thus considerable genotypic heterogeneity and phenotypic heterogeneity; underdiagnosis is common.2 Another form of presentation is seizures, which can lead to a diagnosis of epilepsy and institution of antiepileptic therapy.3
The consequences of undiagnosed LQTS can be catastrophic; if untreated, mortality is significant. Factors indicating poor prognosis include syncope in those aged under 18, female gender, corrected QT interval (QTc) ≥500ms, and a mutation associated with type 2 LQTS.4 Prevention of sudden death is based primarily on beta-blocker therapy, but may also include an implantable cardioverter-defibrillator (ICD).5 In patients refractory to beta-blockers, persistent arrhythmias and repeated automatic shocks cause constant stress and disrupt the patient's social life.6 In such cases, left cardiac sympathetic denervation is a last resort.
Electrical storm is a serious complication in these patients and must be rapidly recognized and treated.
We present the case of a patient diagnosed with epilepsy in childhood and in whom LQTS was diagnosed only in adulthood. Subsequent investigation identified a previously undescribed mutation.
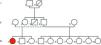
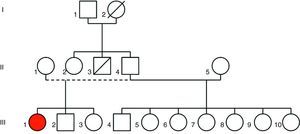
Case reportA 20-year-old black woman had a history of recurrent syncope from the age of two and had been diagnosed with epilepsy. Despite medication with phenobarbital she suffered frequent syncope, sometimes accompanied by tonic seizures, sphincter incontinence and tongue biting. These events were preceded by rapid palpitations and were usually triggered by strong emotions or loud noises. There was a family history of sudden death (a paternal uncle) (Figure 1).
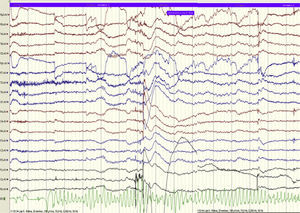
As syncopal episodes persisted, sometimes accompanied by seizures, she was referred to a neurologist for further investigation. The neurological examination was normal, as was the brain magnetic resonance imaging, and the intercritical electroencephalogram showed no evidence of focal slowing or epileptic activity.
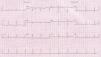
As no abnormalities had been identified on the initial investigation, the patient underwent video electroencephalography and provocation testing, during which she suffered a syncopal episode that was not recorded as epileptic activity on the electroencephalogram. However, the single-lead electrocardiogram (ECG) trace showed polymorphic ventricular tachycardia (torsade de pointes) that coincided with the intravenous administration of saline during provocation testing, and with the syncope (Figure 2).
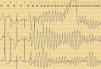
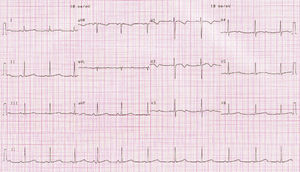
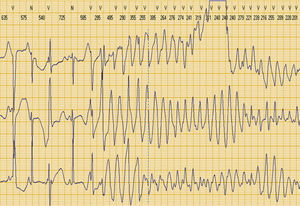
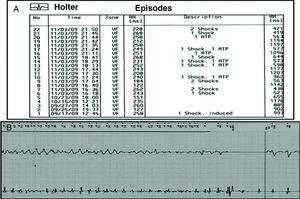
The 12-lead ECG showed a QTc of 482ms (Figure 3). Holter 24-hour monitoring documented frequent ventricular extrasystoles (mean of 30 per hour) and 22 pairs and 8 salvoes of ventricular tachycardia, with two episodes of torsade de pointes, of 30 and 40s; QTc was prolonged, with a mean of 561ms, ranging between 452 and 648ms (Figure 4).
The patient was diagnosed with LQTS and transferred to the cardiology department for monitoring and treatment. Physical examination and transthoracic echocardiogram were normal. Blood samples were taken for genetic study.
Therapeutic management included suspension of phenobarbital, due to its potential for QT interval prolongation, and administration of propanolol in increasing doses up to the maximum tolerated dose. Despite these measures, the patient continued to suffer syncopal episodes preceded by rapid palpitations due to polymorphic ventricular tachycardia, accompanied by mydriasis, tonic movements, urinary incontinence, noisy breathing and sweating. There were no focal neurological signs or post-seizure confusion. Given the failure of beta-blocker therapy at maximum tolerated doses, it was decided to implant an ICD in accordance with current guidelines.12 A Lumax VR® (Biotronik®) VVI-R device was implanted and she was discharged medicated with maximum tolerated beta-blocker therapy.
The patient remained asymptomatic for a month after discharge, but two days after the onset of flu-like symptoms with fever, she had several episodes of rapid palpitations, followed by 13 appropriate shocks and six episodes of antitachycardia pacing, as shown by the ICD event record (Figure 6). Investigation of the etiology of the electrical storm ruled out hypomagnesemia, hypocalcemia, hypokalemia, suspension of current medication, and administration of drugs that prolong the QT interval.
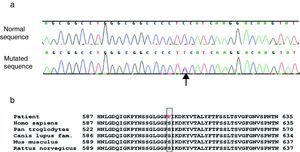
(a) Heterozygous c.1817 C>T p.S606F mutation (arrow) in the KCNH2 gene. The upper line is the sequence from a normal control. (b) Homology of amino acid sequences in different species as tested by the NCBI HomoloGene program (http://www.ncbi.nlm.nih.gov/homologene). Codon p.S606 is homologous in all these species.
Given the fever and clinical suspicion of influenza infection, the patient was screened for H1N1 virus infection, which was confirmed by polymerase chain reaction. Viral myocarditis and other possible complications were excluded. The electrical storm was ended by increasing the ventricular pacing rate, magnesium sulfate perfusion and increased propanolol dose. As her flu symptoms improved it was deemed unnecessary to begin antiviral therapy with oseltamivir, which could have further prolonged the QT interval, and she was discharged. In outpatient consultations, with the patient medicated with propanolol and alprazolam, the ICD was interrogated, and showed several isolated episodes of ventricular tachycardia with appropriate therapies. Propanolol was replaced by atenolol 200mg/day to aid compliance with therapy.
Despite beta-blocker therapy, episodes of ventricular tachycardia/fibrillation requiring cardioversion/defibrillation continued. Left cardiac sympathetic denervation was therefore performed by thoracoscopy, with removal of the lower third of the left stellate ganglion, sympathetic ablation of thoracic ganglia T2 to T5 with resection of the collateral branches. Anhidrosis of the left arm occurred, but not Horner syndrome. On the first postoperative day the atenolol dose was reduced to 100mg/day as the patient remained in sinus rhythm, but on the fourth day there was an episode of syncopal torsade de pointes. The pacing rate was increased to 75bpm and the patient was discharged. At three-month follow-up there had been only four episodes of unsustained polymorphic ventricular tachycardia recorded by ICD home monitoring.
The genetic study revealed a heterozygous mutation, c.1817 C>T, in codon 606 of the KCNH2 gene (Figure 5a), resulting in the replacement of serine by phenylalanine (p.S606F) in the protein coded by KCNH2. The affected amino acid is located at the extracellular level, between the 5th and 6th transmembrane domains of the protein. Three non-pathogenic variants, previously described, were also found in the KCNH2 gene.
Sequencing of the KCNQ1 and SCN5A genes revealed no pathogenic alterations.
The 606 serine residue in the KCNHR protein is highly conserved in various species (Figure 5b). Although not previously been reported in the literature, the cytosine-to-thiamine c.1817 C>T (p.S606F) mutation has been found by a research group at Oxford, UK, in a patient and his mother with “symptoms suggestive of LQTS” (Melanie Proven, personal communication).
Plans are under way for clinical and genetic study of first-degree relatives, which will indicate the degree of segregation of this mutation.
DiscussionThe presentation of LQTS frequently makes it difficult to diagnose. Diagnosis is based on clinical history and the ECG, particularly the QTc interval.1 However, the ECG is not always reliable in this regard, since QT prolongation may not be evident in all leads or may vary between ECGs.3,7
The Schwartz diagnostic score is commonly used. This includes various electrocardiographic characteristics besides QTc interval, such as T-wave morphology, evidence of bradycardia and documented torsade de pointes; clinical criteria include syncope, congenital deafness and a family history of sudden death at young ages, or of LQTS in close relatives.7 Although it has high specificity, the Schwartz score has low sensitivity due to the variable penetrance of the mutations,1 so that some carriers do not present the characteristic phenotype but may still be at risk for ventricular arrhythmias. It is therefore extremely important to perform genetic testing of patients’ families to identify other carriers.
Although genetic study does not dictate diagnosis, it can identify mutations in relevant genes, detecting LQTS in individuals with a non-diagnostic QTc and influencing therapeutic decisions.1 The most common forms of LQTS are associated with mutations in genes coding for potassium channel subunits – KCNQ1 and KCNH2 (also known as hERG, human ether-a-go-go) – in types 1 and 2, and sodium channels (SCN5A), mutations in which are associated with type 3. The latter are also linked to Brugada syndrome.1
As the case reported illustrates, differential diagnosis with epilepsy can be difficult and complex. The seizures seen in LQTS are the consequence of prolonged cerebral hypoperfusion secondary to cardiac arrhythmia. However, Johnson et al. recently demonstrated that seizures are more frequent in type 2 LQTS, which may suggest that they have a common pathophysiological basis.8 Epilepsy may be a part of this subtype of the syndrome, in the same way as deafness in type 1 and gastrointestinal symptoms in type 3.8 The explanation for this phenotype may be that the KCNH2-encoded potassium channel is also expressed in hippocampal astrocytes, which regulate extraneuronal potassium levels; perturbations in these cells may lead to epilepsy.8
There is agreement that the seizure threshold is lowered by cerebral hypoperfusion due to polymorphic ventricular tachycardia and alterations in extraneuronal potassium homeostasis in the hippocampus. It has also been speculated that seizures in LQTS are genuinely epileptic, secondary to disturbances in hippocampal KCNH2 potassium channels, causing temporal lobe epilepsy.8 This is supported by the fact that there are epileptic syndromes clearly associated with mutations in genes coding for sodium and potassium channels, including benign familial neonatal seizures related to KCNQ2 and KCNQ3 potassium channels, and febrile seizures associated with SCN1B and SCN1A sodium channels.9
It has also been suggested that epilepsy can cause malignant arrhythmias. Nashef et al. proposed that sudden unexpected death in epileptic patients may be due to central cardiorespiratory depression during a seizure, leading to ventricular arrhythmias and death.10
No epileptic activity was documented in our patient, even during episodes of arrhythmic syncope. Around 10–40% of patients with epilepsy show no epileptic activity on the EEG, and thus a normal or nonspecific EEG does not rule out a diagnosis of epilepsy.11 Differential diagnosis between LQTS and epilepsy can be challenging, but it is essential: administration of antiepileptic medication can inhibit the potassium channel subunit coded by the KCNH2 gene and thereby increase susceptibility to polymorphic ventricular tachycardia. Several drugs have this effect, including phenobarbital, which was administered to our patient in the case presented.12
LQTS should be considered in young individuals with seizures and an EEG that does not exclude epilepsy. Studies have shown that a third of cases of treatment-resistant epilepsy may be variants of LQTS.13 This highlights the role of 24-hour Holter monitoring and video electroencephalography with ECG, which greatly improve diagnostic accuracy, particularly if the recordings include an arrhythmic event, as in the case presented.
However, the clinical characteristics of syncope remain the central element in diagnosing LQTS, since the arrhythmia is triggered by physiological stress, the type of which is specific to the mutation. Type 1 is most often associated with physical exertion, particularly swimming or diving; type 2 is frequently triggered by sudden loud noises or emotional stress, as in the case described; and in type 3 events occur without emotional arousal at rest or during sleep, without waking the patient.1,8
Correct diagnosis and appropriate treatment can prevent the fatal events that characterize this syndrome. The incidence of SD in LQTS is 1–2% per year and 20% in the first year after diagnosis.14 Beta-blocker therapy reduces events and SD by 70%,15 and is therefore recommended in patients with this diagnosis.5 Over 10% suffer cardiac arrest and SD in spite of treatment;15 ICD implantation is mandatory in those that survive cardiac arrest.5
Arrhythmic risk differs between the three types of LQTS,1,5 being higher in types 2 and 3, and genotype is therefore taken into consideration in indications for ICD implantation.5 The efficacy of beta-blockers also varies between the different types (lower in type 3), again highlighting the need to identify the mutation involved.
In the case presented a previously undescribed mutation, p.S606F, was identified in the KCNH2 (HERG) gene, associated with type 2 LQTS. The fact that this mutation codes for an amino acid in the transmembrane domain that is highly conserved across species renders it pathogenic.
Left cardiac sympathetic denervation is a therapeutic option in LQTS when beta-blocker therapy fails. Reduction of the triggering effect of adrenergic stimulation and modification of the arrhythmogenic substrate (reflected in shorter QTc interval) reduce the number of cardiac events16 at three-month follow-up. However, the patient's QTc interval of 502ms after denervation means the prognosis is less hopeful, with a high likelihood of arrhythmias and SD, as previously suggested by the type 2 genotype and the severity of clinical symptoms before the intervention.
The occurrence of an electrical storm in patients with ICDs is a serious complication. It is essential to exclude factors that prolong the QT interval. Fever, known to trigger arrhythmias in Brugada syndrome, has only occasionally been reported as prolonging QTc in patients with LQTS and only with one mutation (A558P missense mutation in HERG).17 In the case presented, although periods of arrhythmia were documented during the febrile phase, the QTc interval was no longer than at baseline (462ms).
At the same time, the fact that the patient presented an electrical storm when infected with the H1N1 virus raises the possibility that there is a high-risk subgroup of LQTS patients who may be vulnerable to a new arrhythmogenic mechanism specific to the newly identified mutation and H1N1 infection.
ConclusionLQTS is a cause of both syncope and seizures, that makes differential diagnosis with epilepsy essential. However, current knowledge is insufficient to determine whether there is a molecular mechanism that links LQTS and epilepsy.
A new mutation in the KCNH2 gene was identified and described for the first time. This mutation appears to result in susceptibility to electrical storm in the context of fever and viral infection.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Jorge, C. Nova mutação na Síndroma de QT Longo em doente com diagnóstico prévio de epilepsia. doi 10.1016/j.repc.2011.10.003.