Viral myocarditis can lead to heart failure that is refractory to medication. In these cases, a ventricular assist device is a good therapeutic option that can be used as a bridge to transplantation or recovery. We describe the first case in Portugal of recovery with ventricular assistance after severe myocarditis.
Case reportA five-year-old boy with no previous cardiac disease presented with severe viral myocarditis, refractory to medical treatment, with positive serology for parvovirus B19 and Ebstein-Barr virus. A Berlin Heart Excor® was implanted 15 days after diagnosis. A biopsy at the time of implantation showed subendocardial fibrosis. After 40 days of assistance ventricular function recovered and the device was explanted. The patient was discharged from hospital 15 days later.
DiscussionSurvival of children with ventricular assistance has improved significantly because of earlier implantation and coagulation monitoring. The presence of subendocardial fibrosis in the context of myocarditis is not a mandatory indication for transplantation.
A miocardite viral pode cursar com insuficiência cardíaca refractária à medicação. Nestes casos, a assistência ventricular externa é uma alternativa que pode ser usada como ponte para transplante ou para recuperação. Descrevemos o primeiro caso em Portugal de recuperação da função ventricular após assistência ventricular por miocardite grave.
Caso ClínicoApresentamos o caso de uma criança de cinco anos, sexo masculino, sem doença cardíaca prévia, com miocardite viral grave, insuficiência cardíaca refractária à medicação e serologias positivas para Parvovírus B19 e vírus Ebstein-Barr. Foi implantado o Berlin Heart Excor® 15 dias após o diagnóstico. A biopsia cardíaca, na altura da implantação, mostrava áreas de fibrose subendocárdica. Verificou-se recuperação da função miocárdica, tendo sido retirada a assistência ventricular após 40 dias da implantação. O doente teve alta 15 dias depois.
DiscussãoA sobrevida das crianças em assistência ventricular tem vindo a melhorar significativamente, devido ao planeamento atempado e à optimização da anti-coagulação. A presença de fibrose subendocárdica no contexto de miocardite não constitui uma indicação formal para transplante.
Viral myocarditis is rare in children; its real incidence in the general population is unknown. Histologically it is characterized by a mononuclear inflammatory cell infiltrate, interstitial edema and, in some cases, necrosis.1 Its clinical course is mostly benign, with cardiac function recovering spontaneously after a few months, but there can be extensive myocardial necrosis, the necrotic tissue subsequently being replaced by fibrous tissue. The most severe cases progress to dilated cardiomyopathy with heart failure refractory to medication; in such situations a ventricular assist device (VAD) can be used as a bridge to transplantation.
We describe the first case in Portugal of recovery with ventricular assistance after severe myocarditis.
Case reportWe present the case of a five-year-old boy, obese (weight 26kg, height 117cm, BMI >97th percentile), with a history of bronchial asthma.
Three days before admission to the first hospital he presented vomiting, diarrhea, cough and fever. Initial examination revealed gallop rhythm, bilateral rales and hepatomegaly 3cm below the right costal margin. The chest X-ray showed a cardiothoracic index of 65% and diffuse interstitial infiltrate. On echocardiography there was severe left ventricular (LV) dilatation, reduced global contractility, mild mitral regurgitation and pericardial effusion. LV end-diastolic diameter (LVEDD) in parasternal long-axis M-mode was 54mm (z-score=5.5) and LV fractional shortening (LVFS) was 22%. He was admitted and dopamine, milrinone and noradrenaline, furosemide, broad-spectrum antibiotic therapy and invasive mechanical ventilation were begun. During endotracheal intubation he suffered cardiopulmonary arrest and was resuscitated. Digoxin was begun on the third day of hospitalization and a course of levosimendan was begun on the seventh day. Despite this treatment, his condition worsened progressively, with generalized edema and 6-cm hepatomegaly and worsening ventricular function. Etiological study revealed positive serology for parvovirus B19 and Ebstein-Barr virus. His fever persisted despite triple antibiotic therapy.
On the 15th day of hospital stay he was transferred to our center for placement of a VAD as a bridge to heart transplantation due to failure of conventional therapy.
Under inotropic therapy, echocardiography confirmed a highly dilated left ventricle (LVEDD=60mm; z-score=7.5), dilatation of the inferior vena cava (8mm) and moderate mitral regurgitation. LV end-systolic diameter (LVESD) was 49mm (z-score=9) and LV ejection fraction (LVEF) was 30%. Right ventricular (RV) pressure and function were normal.
A Berlin Heart Excor® was implanted, with a 50-ml blood pump, and two 9/12-mm cannulas were introduced into the apical region of the left ventricle and the ascending aorta above the origin of the coronary arteries. The aortic cannula was positioned at an angle of 85° to the aorta. The procedure was performed under cardiopulmonary bypass (103min); the operation lasted three hours and was uneventful. RV function was preserved and there were no signs of RV failure.
The patient was extubated on the fifth day following implantation, without complications, and inotropic support was discontinued on the eighth day.
Rigorous anticoagulation control, which is essential for the correct function of the assist device, was maintained throughout the postoperative period. Intravenous heparin perfusion was begun six hours after implantation at an initial dose of 20U/kg/h, which was then adjusted every six hours during the first week according to activated partial thromboplastin time and platelet count. Thromboelastography was performed and levels of antithrombin III, fibrinogen, d-dimers, platelets, leukocytes and C-reactive protein were assessed daily. Oral anticoagulation with warfarin was begun on the fifth postoperative day and heparin perfusion was discontinued once INR 3.0–3.5 was achieved. There were no thromboembolic or bleeding complications.
With regard to the infection, high fever that was difficult to control persisted until the fifth postoperative day, with positive inflammatory markers. Broad-spectrum antibiotics were administered with a good response. Microbiological exams were all negative. On the 15th postoperative day the fever returned, with positive inflammatory markers. Staphylococcus sanguis was isolated from blood cultures and bronchial secretions and he was treated with antibiotics following antibiotic sensitivity testing, with a good response.
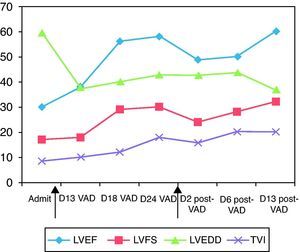
Myocardial biopsy performed at the time of implantation showed moderate subendocardial fibroelastosis but no inflammatory infiltrate or thrombi, but with one focus of myofibroblast proliferation and focal myocytolysis.The following parameters were assessed by serial transthoracic echocardiography: LVEF, LVFS, LVEDD, LVESD, LV outflow tract time/velocity integral, and degree of mitral regurgitation. Progressive improvement in LV contractility was observed (Figure 1); the pump frequency of the VAD was reduced to 30cycles/min and on the 19th day after implantation the patient was transferred to the ward. On the 27th day LV function was assessed with the Berlin Heart Excor® stopped; an increase in cardiac output was seen, due to significantly increased heart rate, and carvedilol was begun. LV function was again assessed with the VAD stopped on the 34th day, with good results. Forty days after implantation, after another course of levosimendan, the Berlin Heart Excor® was explanted electively. The postoperative period was uneventful. He was extubated and inotropic support was withdrawn within 24 hours, and he was transferred back to the ward on the fourth postoperative day. The last echocardiogram before discharge showed LVEF of 60% and LVEDD of 37 mm (z-score=−0.03). The patient was discharged clinically asymptomatic on the 55th day of hospital stay and 15 days after withdrawal of the VAD, medicated with furosemide, spironolactone, aspirin, digoxin, carvedilol and captopril. Three months after discharge he was in NYHA class I, with normal LVEF and being weaned from medication.
Gradual improvement in cardiac function and reduction in left ventricular dilatation after implantation of the ventricular assist device. The arrows indicate the days on which the device was implanted (left) and explanted (right). Admit: day admitted; D13 VAD, D18 VAD, D24 VAD: 13th, 18th, 24th day after implantation of the ventricular assist device; D2 post-VAD, D6 post-VAD, D13 post-VAD: 2nd, 6th, 13th day after explantation of the ventricular assist device. LVEF: left ventricular ejection fraction; LVFS: left ventricular fractional shortening; LVEDD: left ventricular end-diastolic diameter in parasternal long-axis M-mode; TVI: left ventricular outflow tract time/velocity integral.
Viral myocarditis is rare in children and its clinical course is mostly benign, but occasionally it can evolve to severe acute heart failure or to late dilated cardiomyopathy. In such cases, a VAD can act as a last-resort measure, as a bridge to transplantation or recovery. Several devices are available for adults and adolescents, but for children with body surface area of less than 1.20m2 there are only two extracorporeal pulsatile alternatives: the Berlin Heart Excor® (Berlin Heart AG, Berlin, Germany) and the Medos HIA device (Medos Medizintechnik AG, Stolberg, Germany),2 of which the more widely used is the Berlin Heart Excor®. This can be used in single- or biventricular mode; in the former, the pump has two chambers (air and blood) separated by a triple-layer polyurethane membrane, with the air chamber connected to the main apparatus by a tube that transmits the air pressure pulse to move the membrane.3 The blood chamber is connected by two cannulas to the LV apex or the left atrium and the ascending aorta.3
There have been various advances in recent years in adapting VADs for use in pediatric patients, particularly in size of cannulas, chambers and connectors, heparin coating of the blood pump inner surface, and anticoagulant therapy,4 and the technique can now be used at all ages from newborns to adults.3 The throughput of the device can be varied by regulating the pump frequency.
First used in 1990, the Berlin Heart Excor® had been implanted in 500 pediatric patients by May 2009.5
VAD implantation should be carefully planned and every effort should be made to begin ventricular assistance on an urgent rather than emergent basis, before the onset of target-organ dysfunction or cardiogenic shock.6 As myocarditis is generally self-limiting and cardiac function usually recovers, the use of ventricular assistance in children with severe ventricular dysfunction can be an effective and safe solution; the VAD can remain in place for relatively long periods and can even avoid the need for transplantation. Careful anticoagulation control is required to prevent thromboembolic complications, and clinical and laboratory signs of infection must be closely monitored. The timing for weaning from the device is of great importance; cardiac function must be systematically assessed with the device both on and off, and medical therapy must be optimized. If the device is removed too soon, there is a risk of LV failure necessitating a return to ventricular assistance, while prolonging assistance longer than absolutely necessary carries a high risk of infection and thromboembolic events.
In the case presented, although the biopsy showed subendocardial fibrosis, cardiac function recovered. The presence of fibrosis on biopsy is not a mandatory indication for transplantation or a contraindication to the implantation of a ventricular assist device as a bridge to recovery.
Conflicts of interestThe authors have no conflict of interest to declare.
We thank Nuno Raposo of the Cardiothoracic Surgery Department of Hospital de Santa Cruz.
Please cite this article as: Silva, M. Assistência ventricular esquerda numa criança de cinco anos – ponte para recuperação num caso de miocardite viral. Rev Port Cardiol. 2012;31(7-8):521-524.