High plasma levels of low-density lipoprotein (LDL) cholesterol are a risk factor for the development of premature atherosclerosis. Direct adsorption of lipoproteins (DALI) is an apheresis technique by which LDL cholesterol is selectively removed from whole blood.
ObjectiveThe present study describes our experience with DALI LDL apheresis in severely hypercholesterolemic patients.
MethodsThree hypercholesterolemic patients suffering from atherosclerotic complications were treated fortnightly by DALI apheresis, in a total of 308 sessions between December 2008 and January 2013. All patients were on the highest tolerated dose of statins and other lipid-lowering drugs.
ResultsThe sessions were essentially uneventful, adverse events being recorded in only 3.6% of them. A mean 63.3% acute reduction in LDL cholesterol was obtained.
ConclusionsDALI apheresis proved to be a simple, safe and efficient method of lipid apheresis in hypercholesterolemic patients refractory to conservative lipid-lowering therapy.
A hipercolesterolemia manifestada pelos níveis elevados de colesterol das lipoproteínas de baixa densidade constitui um fator de risco major para o desenvolvimento e progressão da doença aterosclerótica prematura.
A adsorção direta de lipoproteínas é uma técnica de LDL-aférese na qual o LDL-c pode ser seletivamente removido do sangue total.
Este trabalho partilha a experiência da LDL-aférese pela técnica de adsorção direta de lipoproteínas no tratamento de doentes com hipercolesterolemia severa.
Três doentes com hipercolesterolemia e doença aterosclerótica documentada foram tratados quinzenalmente com LDL-aférese pela técnica de adsorção direta de lipoproteínas, realizando no total 308 sessões entre dezembro de 2008 e janeiro de 2013. Todos os doentes estavam medicados com estatinas e outros fármacos hipolipemiantes na dose máxima tolerada.
As sessões decorreram na generalidade sem intercorrências. A incidência global de efeitos adversos reportados durante as sessões foi de 3,6%. Em relação aos parâmetros lipídicos obtivemos reduções agudas do colesterol das lipoproteínas de baixa densidade da ordem dos 63,3% (mínimo de 57,3% e máximo de 69,6%).
Em resumo, a LDL-aférese pela técnica de adsorção direta de lipoproteínas demonstrou ser um procedimento simples, seguro e eficaz em doentes com hipercolesterolemia resistentes ao tratamento instituído.
acid citrate dextrose formula A
angiotensin-converting enzyme
apolipoprotein A1
apolipoprotein B 100
autosomal recessive hypercholesterolemia
body mass index
cardiovascular
direct adsorption of lipoproteins
diabetes mellitus
familial hypercholesterolemia
high-density lipoprotein
high-density lipoprotein cholesterol
intermediate density lipoprotein
low-density lipoprotein
low-density lipoprotein cholesterol
low-density lipoprotein receptor
low-density lipoprotein receptor adaptor protein 1
lipoprotein(a)
proprotein convertase subtilisin/kexin type 9
total cholesterol
triglycerides
very-low-density lipoprotein
World Health Organization
Hypercholesterolemia in the form of elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development and progression of coronary artery disease.1
Familial hypercholesterolemia (FH) is a genetic disease of lipoprotein metabolism with autosomal dominant transmission in which affected individuals present very high LDL-C levels, tendon xanthomas and premature coronary artery disease.2
FH is usually due to loss-of-function mutations in the gene encoding the LDL receptor (LDLR), although loss-of-function mutations in the gene encoding apolipoprotein B (ApoB) and gain-of-function mutations in the gene encoding proprotein convertase subtilisin/kexin type 9 (PCSK9) can also cause FH, albeit more rarely.3
Mutations in the LDLR gene lead to absent or reduced production of the protein depending on the type of mutation; mutations in the APOB gene encode mutant ApoB 100 molecules, which have less affinity for LDLR, thus blocking or reducing the binding of ApoB to LDLR, while gain-of-function missense mutations in the PCSK9 gene enhance LDLR catabolism.4–7
Homozygous FH is extremely rare, with a prevalence of 1/1000000. Most cases of homozygosity involve either the same functional mutation in both alleles (true homozygotes) or different functional mutations in each allele (compound heterozygotes).
Another rare condition that presents the same phenotype as homozygous FH is autosomal recessive hypercholesterolemia (ARH), which is caused by mutations in the gene encoding LDLR adaptor protein 1 (LDLRAP1) and the parents do not usually present dyslipidemia.8
There are also rare cases of double heterozygosity, which present a more severe phenotype than patients with heterozygous FH but less severe than those with homozygous FH.9–11
Heterozygous FH is much more frequent than homozygous FH, with a prevalence of 1/300–500 individuals; it is thus one of the most common severe genetic diseases and is recognized as a public health problem by the World Health Organization (WHO),12,13 who estimate that over 10000000 individuals worldwide have FH. However, less than 10% are diagnosed and of these, less than 25% receive lipid-lowering therapy.13
Treatment of dyslipidemia, for both primary and secondary prevention, focuses on reducing LDL-C. Numerous clinical trials have demonstrated the benefits of statin therapy in reducing LDL-C and cardiovascular (CV) morbidity and mortality.1,14
Among the non-pharmacological treatment options for severely hypercholesterolemic patients who are intolerant or refractory to lipid-lowering therapy is LDL apheresis. There are various techniques, all of which lead to significant and acute reduction in plasma LDL-C levels.15 At Hospital Santo António, Porto, we use the technique of direct adsorption of lipoproteins (DALI).
ObjectiveWe describe the experience of the multidisciplinary clinic for lipid disorders assessing severely hypercholesterolemic patients refractory to lipid-lowering therapy who underwent DALI LDL apheresis.
MethodsSince 1993 the endocrinology department of Hospital Santo António has had a multidisciplinary clinic for lipid disorders, which includes specialists in endocrinology and nutrition and has a joint working protocol with the cardiology, neurology, clinical chemistry and nephrology departments. There is a weekly clinic whose main function is the study and treatment of severe lipid disorders, and DALI LDL apheresis has been available there since 2008.
Direct adsorption of lipoproteinsIn this technique, whole blood is passed through an adsorber containing polyacrylamide gel. This consists of polyanions with negatively charged carboxylate groups that interact selectively with the cationic groups of Apo B found in very low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), LDL and lipoprotein(a) (Lp(a)). Each of these lipoproteins contains a single molecule of Apo B, by which the lipoproteins are immobilized in the adsorber through electrochemical interactions.
High-density lipoproteins (HDL) do not interact with the polyanions in the adsorber since they are coated with Apo A1, and are thus not eliminated by the technique.
After passing through the adsorber, the blood depleted of all Apo B-containing lipoproteins is returned to the patient. Reductions in LDL-C of over 60% have been observed.16,17
The apheresis sessions were performed using the following equipment from Fresenius Medical Care, Germany: 4008 ADS monitor, DALI adsorbers, priming solution and acid citrate dextrose formula A (ACD-A). According to the German clinical guidelines, the aim of LDL apheresis is to achieve a reduction in LDL-C of ≥60% per session. Two configurations are available: DALI 750 and DALI 1000.18
In patients indicated for prolonged or chronic treatment, the creation of an arteriovenous fistula is recommended in order to facilitate vascular access.
The volume of blood processed per session is tailored to the patient's gender and weight, and flow rate varies between 60 and 100 ml/min.
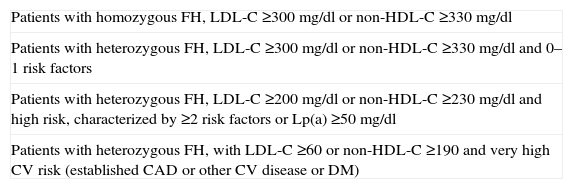
A patient is referred for LDL apheresis following agreement by the multidisciplinary team based on the criteria recommended by the US Food and Drug Administration (Table 1).19
Selection criteria for LDL apheresis recommended by the US Food and Drug Administration.
| Patients with homozygous FH, LDL-C ≥300 mg/dl or non-HDL-C ≥330 mg/dl |
| Patients with heterozygous FH, LDL-C ≥300 mg/dl or non-HDL-C ≥330 mg/dl and 0–1 risk factors |
| Patients with heterozygous FH, LDL-C ≥200 mg/dl or non-HDL-C ≥230 mg/dl and high risk, characterized by ≥2 risk factors or Lp(a) ≥50 mg/dl |
| Patients with heterozygous FH, with LDL-C ≥60 or non-HDL-C ≥190 and very high CV risk (established CAD or other CV disease or DM) |
DM: diabetes mellitus; CV: cardiovascular; FH: familial hypercholesterolemia; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; Lp(a): lipoprotein(a).
LDL apheresis is recommended for these patients if they have not attained therapeutic targets after six months of non-pharmacological or pharmacological therapy at the maximum tolerated dose.
Laboratory testsLaboratory tests are performed by the clinical chemistry department of Hospital Santo António using a Cobas Integra 800 analyzer (Roche Diagnostics) and appropriate reagents.
Blood samples are obtained directly via the afferent line immediately before the start of each session. Samples are collected via the same line at the end of treatment before the extracorporeal circuit is rinsed with saline.
Measurement of lipid parametersAcute percentage reductions in lipid parameters in a single treatment are calculated according to the following formula: 100×(pre-session value−post-session value)/pre-session value.
Long-term reductions in each lipid parameter are calculated from the baseline value prior to the first session, the mean value after the antepenultimate session (post-session n-2), before the penultimate session (pre-session n-1), after the penultimate session (post-session n-1) and before the last session (pre-session n),17,20 according to the following formulas:
- •
Long-term percentage reduction in lipid parameters: 100×(baseline value−mean value)/baseline value
- •
Mean value of lipid parameters: (post-session value n-2+pre-session value n-1+post-session value n-1+pre-session value n)/4.
n-2, n-1 and n refer to the last three sessions in chronological order from the earliest to the last, respectively.
The same formulas were used for all lipid parameters: LDL-C, triglycerides (TG), total cholesterol (TC), HDL-C and non-HDL-C, the latter being calculated as the difference between TC and HDL-C.
All patients underwent fortnightly sessions of DALI LDL apheresis lasting an average of 90 min while maintaining previous pharmacological therapy.
Patient 1A 55-year-old man had been diagnosed with asymptomatic ischemic heart disease on exercise testing at age 37, followed by stenting in 1995 for critical stenosis of the right coronary artery and placement of two further stents in 2010 due to restenosis of the same artery.
He had no CV risk factors including smoking, diabetes mellitus (DM), hypertension, family history of premature ischemic heart disease or dyslipidemia and was not overweight (body mass index [BMI] 23.8 kg/m2).
His pre-treatment lipid profile was TC 390 mg/dl, TG 140 mg/dl, HDL-C 50 mg/dl, LDL-C 312 mg/dl, Apo A1 109 mg/dl and Apo B 186 mg/dl, with no other abnormalities on laboratory tests.
Since the patient presented sustained elevation of transaminases under therapy with rosuvastatin 40 mg/day, intolerance to resins manifested as constipation and severe flushing with nicotinic acid, he was medicated with rosuvastatin 20 mg/day (maximum tolerated dose) and ezetimibe 10 mg/day. He was also taking diltiazem 120 mg, clopidogrel 75 mg and aspirin 100 mg. However, his dyslipidemia was refractory to maximum tolerated pharmacological therapy.
Genetic study showed a heterozygous functional mutation in the PCSK9 gene.
He began LDL apheresis as adjuvant therapy in December 2008 using the DALI 750 filter, while maintaining his usual lipid-lowering therapy.
Four years after beginning LDL apheresis, carotid Doppler ultrasound showed diffuse carotid atherosclerosis with mild stenosis (<20%) of the right and left internal carotid arteries; myocardial perfusion study revealed no evidence of ischemia, a mild fixed inferolateral defect and good left ventricular global systolic function.
Patient 2A 59-year-old man had been diagnosed with severe dyslipidemia in routine laboratory tests at the age of 36. He had xanthelasmas and tendon xanthomas and asymptomatic carotid atherosclerosis, with >70% stenosis of the right internal carotid artery and 50–70% stenosis of the left internal carotid artery. He underwent elective endarterectomy of the right internal carotid artery in March 2007.
He was a former smoker, of normal weight (BMI 22.8 kg/m2), and had a family history of dyslipidemia (mother, siblings and daughter with hypercholesterolemia) and no other CV risk factors.
His pre-treatment lipid profile was TC 378 mg/dl, TG 75 mg/dl, HDL-C 112 mg/dl, LDL-C 251 mg/dl, Apo A1 223 mg/dl and Apo B 176 mg/dl, with no other abnormalities on laboratory tests.
Since the patient experienced myalgia on rosuvastatin 40 mg/day, intolerance to resins at the maximum recommended dose and worsening gastric disease (peptic ulcer) with nicotinic acid, he was medicated with cholestyramine 8 g/day, rosuvastatin 20 mg/day and ezetimibe 10 mg/day, as well as aspirin 325 mg, valsartan 160 mg and esomeprazole 40 mg.
Genetic study showed a heterozygous functional mutation in the LDLR gene.
He began LDL apheresis in March 2009 using the DALI 750 configuration, while maintaining his previous lipid-lowering therapy.
Fours years after beginning LDL apheresis, carotid Doppler ultrasound showed diffuse atherosclerosis with mild stenosis (<20%) of the right internal carotid artery and moderate stenosis of the left internal carotid artery, with an echogenic, partially calcified atheromatous plaque causing 30–50% stenosis but with normal carotid flow. Myocardial perfusion study was suggestive of mild ischemia in the territory of the anterior descending artery, but with good left ventricular global systolic function.
Patient 3A 62-year-old woman with severe symptomatic aortic stenosis underwent aortic valve replacement with a mechanical prosthesis in July 2010. She also had asymptomatic carotid arteriosclerosis, atheromas in the common carotid arteries and proximal internal carotid arteries with <30% stenosis.
She had a family history of dyslipidemia (daughter and grandson with hypercholesterolemia), was of normal weight (BMI 22.7 kg/m2) and had no other CV risk factors (non-smoker, no hypertension or DM and no family history of premature CV disease).
Her pre-treatment lipid profile was TC 379 mg/dl, TG 71 mg/dl, HDL-C 67 mg/dl, LDL-C 298 mg/dl, Apo A1 120 mg/dl and Apo B 184 mg/dl, with no other abnormalities on laboratory tests.
Since the patient was intolerant to rosuvastatin 40 mg and resins, she was medicated with rosuvastatin 20 mg, nicotinic acid 1 g (maximum tolerated), ezetimibe 10 mg, warfarin 5 mg, aspirin 100 mg and diazepam 5 mg.
Genetic study showed a heterozygous functional mutation in the LDLR gene and another mutation of unknown significance in the same gene; functional studies are in progress to assess the pathogenicity of this alteration, and if confirmed, the patient will be classified as a compound heterozygote.
She began LDL apheresis in March 2009, while maintaining her usual lipid-lowering therapy. Due to the lack of adequate response to the DALI 750 configuration, the DALI 1000 filter was subsequently used.18
Four years after beginning LDL apheresis, carotid Doppler ultrasound showed an atheroma with regular contours in the right internal carotid artery with <30% stenosis and <20% stenosis in the left internal carotid artery; myocardial perfusion study was within normal limits, with good left ventricular global systolic function.
ComplicationsBetween December 2008 and January 2013, 308 treatments were performed (Tables 2–6), during which there were 11 complications – 10 minor and one major – including six episodes of chest pain, three of hypotension (one accompanied by an anaphylactic reaction) and one of clotting of the extracorporeal circuit.
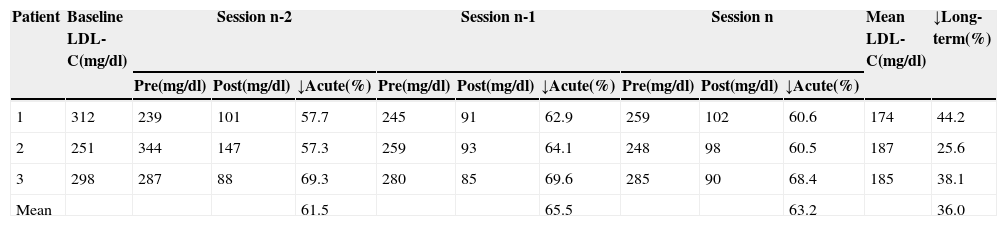
Acute and long-term reductions in low-density lipoprotein cholesterol by direct adsorption of lipoprotein LDL apheresis.
| Patient | Baseline LDL-C(mg/dl) | Session n-2 | Session n-1 | Session n | Mean LDL-C(mg/dl) | ↓Long-term(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | ||||
| 1 | 312 | 239 | 101 | 57.7 | 245 | 91 | 62.9 | 259 | 102 | 60.6 | 174 | 44.2 |
| 2 | 251 | 344 | 147 | 57.3 | 259 | 93 | 64.1 | 248 | 98 | 60.5 | 187 | 25.6 |
| 3 | 298 | 287 | 88 | 69.3 | 280 | 85 | 69.6 | 285 | 90 | 68.4 | 185 | 38.1 |
| Mean | 61.5 | 65.5 | 63.2 | 36.0 | ||||||||
LDL-C: low-density lipoprotein cholesterol. n-2, n-1 and n refer to the last three sessions; pre and post: values before and after treatment sessions; ↓Acute: acute reduction; ↓Long-term: long-term reduction.
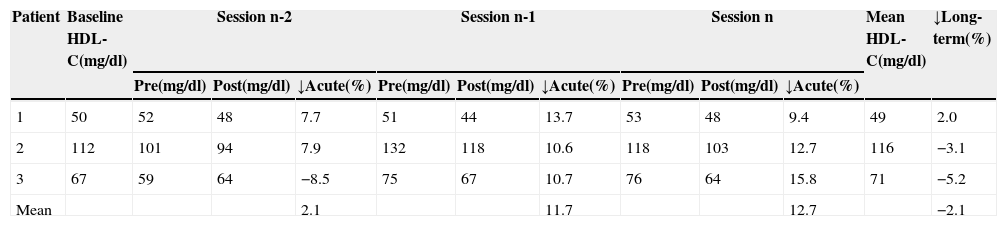
Acute and long-term reductions in high-density lipoprotein cholesterol by direct adsorption of lipoprotein LDL apheresis.
| Patient | Baseline HDL-C(mg/dl) | Session n-2 | Session n-1 | Session n | Mean HDL-C(mg/dl) | ↓Long-term(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | ||||
| 1 | 50 | 52 | 48 | 7.7 | 51 | 44 | 13.7 | 53 | 48 | 9.4 | 49 | 2.0 |
| 2 | 112 | 101 | 94 | 7.9 | 132 | 118 | 10.6 | 118 | 103 | 12.7 | 116 | −3.1 |
| 3 | 67 | 59 | 64 | −8.5 | 75 | 67 | 10.7 | 76 | 64 | 15.8 | 71 | −5.2 |
| Mean | 2.1 | 11.7 | 12.7 | −2.1 | ||||||||
HDL-C: high-density lipoprotein cholesterol. n-2, n-1 and n refer to the last three sessions; pre and post: values before and after treatment sessions; ↓Acute: acute reduction; ↓Long-term: long-term reduction.
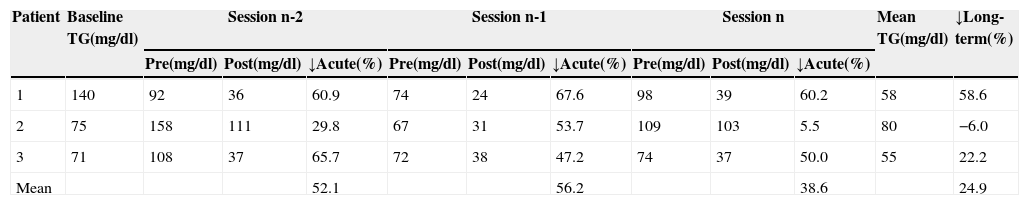
Acute and long-term reductions in triglycerides by direct adsorption of lipoprotein LDL apheresis.
| Patient | Baseline TG(mg/dl) | Session n-2 | Session n-1 | Session n | Mean TG(mg/dl) | ↓Long-term(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | ||||
| 1 | 140 | 92 | 36 | 60.9 | 74 | 24 | 67.6 | 98 | 39 | 60.2 | 58 | 58.6 |
| 2 | 75 | 158 | 111 | 29.8 | 67 | 31 | 53.7 | 109 | 103 | 5.5 | 80 | −6.0 |
| 3 | 71 | 108 | 37 | 65.7 | 72 | 38 | 47.2 | 74 | 37 | 50.0 | 55 | 22.2 |
| Mean | 52.1 | 56.2 | 38.6 | 24.9 | ||||||||
TG: triglycerides. n-2, n-1 and n refer to the last three sessions; pre and post: values before and after treatment sessions; ↓Acute: acute reduction; ↓Long-term: long-term reduction.
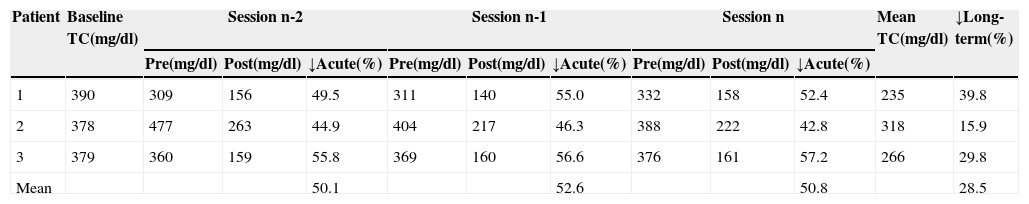
Acute and long-term reductions in total cholesterol by direct adsorption of lipoprotein LDL apheresis.
| Patient | Baseline TC(mg/dl) | Session n-2 | Session n-1 | Session n | Mean TC(mg/dl) | ↓Long-term(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | ||||
| 1 | 390 | 309 | 156 | 49.5 | 311 | 140 | 55.0 | 332 | 158 | 52.4 | 235 | 39.8 |
| 2 | 378 | 477 | 263 | 44.9 | 404 | 217 | 46.3 | 388 | 222 | 42.8 | 318 | 15.9 |
| 3 | 379 | 360 | 159 | 55.8 | 369 | 160 | 56.6 | 376 | 161 | 57.2 | 266 | 29.8 |
| Mean | 50.1 | 52.6 | 50.8 | 28.5 | ||||||||
TC: total cholesterol. n-2, n-1 and n refer to the last three sessions; pre and post: values before and after treatment sessions; ↓Acute: acute reduction; ↓Long-term: long-term reduction.
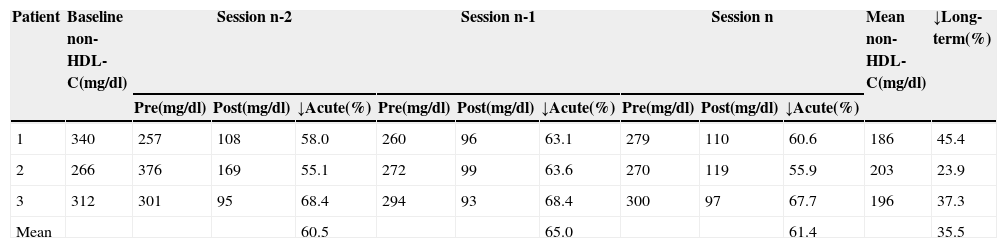
Acute and long-term reductions in non-high-density lipoprotein cholesterol by direct adsorption of lipoprotein LDL apheresis.
| Patient | Baseline non-HDL-C(mg/dl) | Session n-2 | Session n-1 | Session n | Mean non-HDL-C(mg/dl) | ↓Long-term(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | Pre(mg/dl) | Post(mg/dl) | ↓Acute(%) | ||||
| 1 | 340 | 257 | 108 | 58.0 | 260 | 96 | 63.1 | 279 | 110 | 60.6 | 186 | 45.4 |
| 2 | 266 | 376 | 169 | 55.1 | 272 | 99 | 63.6 | 270 | 119 | 55.9 | 203 | 23.9 |
| 3 | 312 | 301 | 95 | 68.4 | 294 | 93 | 68.4 | 300 | 97 | 67.7 | 196 | 37.3 |
| Mean | 60.5 | 65.0 | 61.4 | 35.5 | ||||||||
HDL-C: high-density lipoprotein cholesterol. n-2, n-1 and n refer to the last three sessions; pre and post: values before and after treatment sessions; ↓Acute: acute reduction; ↓Long-term: long-term reduction.
Similar to reports in the literature, 96.4% of the sessions were uneventful.21 The overall incidence of adverse events was 3.6%, also similar to that reported by other authors, and treatment had to be suspended in only one case.21 No prolonged or irreversible adverse effects were seen in any patient.
Comparing the adverse effects in our patients with those observed by Bosch et al.,21 the most common adverse effect in our population was angina (1.95% vs. 0.09%), followed by hypotension (0.97% vs. 0.97%), clotting of the extracorporeal circuit (0.32% vs. 0.03%) and anaphylactic reaction (0.32% vs. 0%).
There were six episodes of angina during the 308 LDL apheresis sessions, a higher percentage than that reported in the literature.21 These all occurred in the same patient, who had critical aortic valve stenosis, and were reversed by reducing flow rate and administering fluids, oxygen and nitrates. The etiology of the angina was probably multifactorial but we believe the underlying aortic stenosis was the main factor, since following surgical treatment, there were no further episodes of angina.
Hypotension is reported as the most common complication of LDL apheresis.16,20,21 This may be due to hypovolemia, vasodilation or vasovagal reaction. In the DALI technique, the negatively charged surfaces of the adsorber increase concentrations of factor XII, kallikrein and bradykinin. Bradykinin can cause hypotension, flushing and Quincke's edema. Although it is generated in the adsorber and reinfused into the patient, it is rapidly degraded by angiotensin-converting enzyme (ACE), so levels in the pre-adsorber afferent line are negligible.16,22
The main cause of hypovolemia during DALI apheresis appears to be the initial transfer of blood to the extracorporeal circuit. Like other authors, we replace part of the initial blood volume with saline solution in the contralateral vein while the extracorporeal circuit is filling in order to minimize this effect and prevent hypotension.16
One of the three cases of hypotension was more severe and accompanied by pallor, dyspnea and a feeling of imminent death. This was in a patient who had been prescribed ramipril for the first time and took it inadvertently on the day of the LDL apheresis session. This episode was interpreted as an anaphylactic reaction. The DALI technique can cause severe hypotension in patients taking ACE inhibitors due to decreased bradykinin catabolism through ACE inhibition and the increased bradykinin production associated with the procedure. Drugs that inhibit degradation of bradykinin into inactive metabolites are thus contraindicated in patients undergoing DALI LDL apheresis.21,23
In order to avoid the risk of hypotension in patients undergoing DALI apheresis, ACE inhibitors should be suspended 24 hours before treatment and whenever possible replaced by other antihypertensive drugs, such as angiotensin receptor blockers, that do not inhibit ACE.
Of the three cases of hypotension, two were resolved with intravenous fluid therapy and reduction of flow rate; in the more severe case, LDL apheresis was suspended and oxygen, fluid and intravenous corticosteroid therapy were required.
As described in the literature, 1 ml of ACD-A per 20–40 ml of blood was used to prevent clotting of the extracorporeal circuit, which proved adequate for anticoagulation.20,21 In 308 sessions, there was only one episode of clotting of the extracorporeal circuit (0.32% vs. 0.17%).20
Besides lipoproteins, the DALI column also adsorbs positively charged ions such as calcium and magnesium. We observed no hypocalcemia or hypomagnesemia in our experience, but like other authors we used a priming solution that contains these electrolytes, saturating the column with these cations and thus preventing hypocalcemia and hypomagnesemia during treatment; in addition, an ampoule of calcium gluconate was used prophylactically at the end of each session.24
Iron deficiency, leading to microcytic anemia, has also been reported in patients under chronic LDL apheresis. This may be due to frequent laboratory tests and residual blood loss in the extracorporeal system after each session. No iron deficiency or anemia was observed in our patients.
We used the methodology previously described by us and other authors17,20 to assess the technique's efficacy in terms of lipid parameters.
Mean acute reductions in LDL-C of 63.3% (57.3–69.6%) were obtained, which are similar to those described by other authors and meet the objectives stated in the German clinical guidelines for LDL apheresis.18,21
With regard to long-term reductions in LDL-C, we achieved a mean reduction of 36.0% (25.6–44.2%), slightly lower than reported in the literature21; however, we believe that the difference may be explained by the longer treatment time and larger number of patients, as well as better patient compliance with drug therapy, in these studies.
We obtained a mean acute reduction in non-HDL-C of 62.3% (55.1–68.4%) and a mean long-term reduction of 35.5% (23.9–45.4%), comparable to those seen for LDL-C. We believe that this was due to the characteristics of our patients’ dyslipidemia: hypercholesterolemia but no DM, obesity or metabolic syndrome criteria, and hence no factors that promote the production of VLDL or IDL. On the other hand, it may have been due to TG values of <400, as reflected in a low error rate in calculating LDL-C by the Friedewald formula.25
Acute reductions in HDL-C were similar to those obtained by other authors and are related to low TG levels, which in turn depend heavily on the food ingested prior to treatment sessions. No significant long-term changes in HDL-C were observed, which demonstrates the selectivity of DALI LDL apheresis.
ConclusionThe results reflect the efficacy and selectivity of DALI LDL apheresis and are comparable to those obtained in previous studies of the technique.20,21
In 308 sessions over a 49-month period the technique was shown to be safe, effective and selective for LDL-C, with acute reductions of over 60% and no significant changes in other lipoproteins. The main objective of LDL-C reduction is to prevent the development and progression of atherosclerosis, and the improvement seen in our patients’ lipid profiles was reflected in the absence of disease progression. The selective elimination of LDL-C by DALI apheresis is an important advance in the treatment of hypercholesterolemic patients with high CV risk.26
LDL apheresis in conjunction with lipid-lowering therapy prevented the development of atherosclerosis in patients with severe dyslipidemia and high CV risk.
The low incidence of complications means that LDL apheresis is well tolerated by patients, which is reflected in the high rate of adhesion to the treatment.
DALI LDL apheresis was shown to be a simple, safe and effective treatment in patients with FH refractory to diet modification and pharmacological therapy and should be considered as an adjuvant therapy for hypercholesterolemic patients who are intolerant or refractory to statin therapy and for those with contraindications to drug therapy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Dr. Mafalda Bourbon for her help with this article and with genetic testing.
Please cite this article as: Palma I, Caldas AR, Palma IM, Queirós JA, Madureira A, Oliveira JC, et al. LDL-aférese no tratamento de hipercolesterolemia familiar: experiência do Hospital Santo António – CHP. Rev Port Cardiol. 2015;34:163–172.