Until the development of drug-eluting stents (DES), diffuse in-stent restenosis (ISR) was the main limitation of bare-metal stents in percutaneous coronary intervention (PCI). Among the different treatments available, intracoronary brachytherapy (BT) emerged as one of the most promising, although it was almost abandoned with the increasing use of DES.
ObjectiveTo assess the Portuguese experience with 90Sr/90Y beta brachytherapy for the treatment of diffuse ISR regarding long-term (>10 years) major adverse cardiac events (MACE) and angiographic restenosis.
MethodsThis single-center, retrospective, observational study included 12 consecutive patients treated between January and June 2001, mean age 58.6±9.9 years (range 43–77 years), 11 male. All had chronic stable angina, 75% had dyslipidemia, 58% had hypertension, 50% had peripheral arterial disease, 42% had diabetes and 50% had multivessel disease. Recurrent ISR was present in half of the patients and 11 had normal left ventricular function. After balloon dilatation, BT was performed using an 90Sr/90Y (Novoste Beta-Cath™) beta radiation source. All patients remained under dual antiplatelet therapy until scheduled nine-month follow-up angiography. Patients were followed for the occurrence of death (all-cause and cardiovascular), non-fatal myocardial infarction (MI), revascularization, stent thrombosis and angiographic restenosis. MACE were defined as the combined incidence of cardiac death, MI and urgent target vessel revascularization.
ResultsIn all cases there was both clinical and angiographic success. In a mean follow-up of 10.9±2.5 years, 19 events occurred in seven patients: death in three (25%), only one cardiac (8.3%); ST-elevation MI in one (related to a non-target vessel) (8.3%); and 15 revascularizations in five (42%), of which nine were of the target vessel (mainly in the first two years). There was only one case of probable stent thrombosis. Angiographic restenosis at nine months was 27% (three out of 11 patients), of which two were total occlusions. Ten-year MACE-free survival was 42% (5 patients).
ConclusionsIntracoronary beta brachytherapy for the treatment of diffuse ISR in this small cohort of patients proved to be safe and efficacious, with no late adverse events related to intracoronary radiation.
No advento dos stents eluidores de fármaco (DES), a reestenose difusa (>20 mm) intrastent (RDIS) constituía a principal limitação ao uso de stents metálicos na intervenção coronária (PCI). Das várias soluções propostas para o seu tratamento, a braquiterapia intracoronária (BT) revelou-se uma das mais eficazes, sendo praticamente abandonada após a introdução dos DES.
ObjetivoAvaliar os resultados clínicos a longo prazo (>10 anos) do uso de BT beta com 90Sr/Y90 para o tratamento RDIS, em termos de eventos cardíacos major (MACE) e reestenose angiográfica.
População e métodosEstudo retrospetivo observacional de centro único incluindo 12 doentes (dts) consecutivos entre janeiro e julho de 2001, 11 homens, com a idade média de 58,6±9,9 anos (43-77 anos). Todos tinham angor estável. Os principais fatores de risco eram dislipidemia (75%), HTA (58%), doença arterial periférica (50%) e diabetes (42%). Havia doença multivaso em seis dts, tratava-se da 2.a ou 3.a reestenose em seis dts e a função ventricular era normal em 11. A BT foi feita após dilatação com balão, usando a fonte emissora de radiação beta 90Sr/Y90 (Beta-Cath™/Novoste). Todos permaneceram sob dupla antiagregação pelo menos até à angiografia de controlo, programada para todos aos nove meses. Foi considerada a ocorrência de morte global e cardiovascular, enfarte do miocárdio não fatal (EAM), revascularização coronária, trombose de stent e reestenose angiográfica. Foi avaliada a incidência do evento hierárquico combinado (MACE) de: morte cardiovascular, EAM, revascularização coronária urgente do vaso alvo (TVRurg).
ResultadosVerificou-se sucesso angiográfico e clínico em 100% dos casos.
No seguimento médio de 10,9±2,5 anos, ocorreram 19 eventos em sete dts: morte em três dts (25%), apenas uma cardíaca (8,3%), EAM com elevação de ST (vaso não alvo) num dt (8,3%) e 15 novas revascularizações em cinco dts (42%): nove relacionadas com o vaso alvo (sobretudo nos primeiros dois anos). Apenas se verificou uma trombose de stent, provável. A reestenose angiográfica aos nove meses de seguimento foi de 27% (3/11 dts) com duas oclusões. A taxa de sobrevivência livre de MACE aos dez anos foi de 42% (cincodts).
ConclusõesA braquiterapia com radiação beta para RDIS, neste pequeno grupo de dts, demonstrou-se eficaz e segura, sem eventos tardios atribuíveis à radiação intracoronária.
Before the drug-eluting stent (DES) era, restenosis due to tissue proliferation in the neointima was the main limitation to wider use of bare-metal stents (BMS) in percutaneous coronary intervention (PCI). In their angiographic classification of in-stent restenosis (ISR), Mehran et al.1 described diffuse ISR as having higher rates of recurrence and need for subsequent revascularization. Of the various methods used to control in-stent neointimal hypoplasia, intracoronary brachytherapy showed the strongest antiproliferative effect2–6 and the best results, and its safety and efficacy were demonstrated in various randomized trials using gamma and beta radiation.6–11 Following its approval by the US Food and Drug Administration in November 2000, the technique became established as an adjuvant to balloon angioplasty for the treatment of ISR and was incorporated into the guidelines on PCI.12,13 However, the long-term efficacy of brachytherapy has been questioned,14 and there have been few studies with long-term follow-up after beta brachytherapy.15–20
The aim of the present study is to analyze the experience of the only Portuguese center using beta brachytherapy (in 2001) and to assess its late efficacy and safety in long-term follow-up (>10 years).
MethodsThe study population consisted of 12 consecutive patients who underwent beta brachytherapy between January and July 2001 for diffuse ISR in BMS, defined as ≥50% stenosis and ≥20 mm in length.
Patients’ mean age was 58.6±9.9 years (range 43–77 years), and 11 were male. The main cardiovascular risk factors were dyslipidemia in nine patients (75%), hypertension in seven (58%), and diabetes in five (42%). Six patients (50%) had concomitant peripheral arterial disease. All had chronic stable angina; four (33%) had previous myocardial infarction (MI) and three (25%) had previous coronary artery bypass grafting (CABG). There was multivessel disease in eight patients, and only one had left ventricular dysfunction. It was the first occurrence of ISR in six patients, the second in four, and the third in two. Only one case was proliferative ISR. All patients were under maximal tolerated medication with aspirin, statins, nitrates, beta-blockers, calcium channel blockers and angiotensin-converting enzyme inhibitors (Table 1).
Baseline characteristics of the study population.
| Patients (n) | 12 |
| Age (years) | 58.6±9.9 |
| Male, n (%) | 11 (92) |
| Risk factors | |
| Diabetes, n (%) | 5 (42) |
| Dyslipidemia, n (%) | 9 (75) |
| Hypertension, n (%) | 7 (58) |
| PAD, n (%) | 6 (50) |
| Smoking, n (%) | 2 (17) |
| History | |
| MI, n (%) | 4 (33) |
| CABG, n (%) | 3 (25) |
| Clinical presentation | |
| Stable angina, n (%) | 12 (100) |
| Positive ischemia test, n (%) | 10 (83) |
| Multivessel disease, n (%) | 8 (67) |
| Preserved LV function, n (%) | 11 (92) |
| Indication for PCI | |
| First restenosis, n (%) | 6 (50) |
| Second restenosis, n (%) | 4 (33) |
| Third restenosis, n (%) | 2 (17) |
| Medication before intervention | |
| Aspirin, n (%) | 12 (100) |
| Statins, n (%) | 9 (75) |
| Beta-blockers, n (%) | 9 (75) |
| Calcium channel blockers, n (%) | 9 (75) |
| ACE inhibitors, n (%) | 7 (58) |
| Nitrates, n (%) | 10 (83) |
ACE: angiotensin-converting enzyme; CABG: coronary artery bypass grafting; LV: left ventricular; MI: myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention.
Quantitative analysis of the coronary angiograms was performed with the CAAS II™ cardiovascular angiographic analysis system, using the diameter of the guiding catheter as a reference. The analysis included the reference vessel diameter, lesion length, degree of stenosis and minimal luminal diameter. Angiographic restenosis was defined as intraluminal ISR of ≥50%. The length of the lesions (40–90 mm) and the small diameter of the vessels treated (mean 2.5 mm) should be noted (Table 2).
Characteristics of the lesions treated.
| Target vessel n (%) | |
| Anterior descending | 4 (33) |
| Circumflex | 2 (17) |
| Right coronary | 6 (50) |
| Type of stent (total 20), n (%) | |
| ACS MULTI-LINK™ TETRA (Guidant/Advanced Cardiovascular Systems) | 8 (40) |
| Bx Velocity™ (Cordis, Johnson & Johnson) | 7 (35) |
| AVE GFX-2™ (Arterial Vascular Engineering) | 2 (10) |
| AVE S670™ (Arterial Vascular Engineering) | 1 (5) |
| NIROYAL™ (Medinol/Scimed Life Systems) | 1 (5) |
| Bx Velocity™ heparin-coated stent (Cordis, Johnson & Johnson) | 1 (5) |
| Quantitative analysis | |
| Mean reference vessel diameter (mm) | 2.56±0.58 |
| Mean minimal luminal diameter (mm) | 1.0±0.12 |
| Mean degree of stenosis (%) | 64.4±16.9 |
| Mean lesion length (mm) | 55.9±19.5 (40–90 mm) |
Brachytherapy was performed using a 90Sr/90Y beta radiation source (Novoste Beta-Cath™). Beta radiation was chosen because, unlike gamma radiation, it did not require logistical changes to our catheterization laboratory. A protocol was established with the Francisco Gentil Portuguese Oncology Institute in Lisbon, including radiation therapists and medical physicists. All patients gave their informed consent and the treatment was approved by the ethics committee of Hospital de Santa Cruz.
All procedures were performed via femoral access using 8F guide catheters. Following control angiography and confirmation of ISR, the diagnostic catheter was replaced by the angioplasty guiding catheter and the lesion was crossed using a 0.014″ guidewire. The restenosed vessel was dilated with a balloon, the diameter and length of which were selected according to vessel diameter and lesion length. The 5F Beta-Cath™ delivery catheter has three lumens, one for the guidewire, one for delivery of the radiation source, and one to deliver saline solution. All catheters used were 40 mm in length. The catheter was advanced over the guidewire, radiation being applied from the distal to the proximal portion of the lesion, in all cases extending radiation coverage to the adjacent tissue without angiographically detectable disease. One or more applications were performed, depending on lesion length (one application in seven patients, two in three and three in two). Radiation dose and time varied in accordance with predefined tables for vessel and stent diameter (18.4 Gy for diameters ≥2.7 and <3.35 mm with radiation time 215 s and 23.0 Gy for diameters >3.35 and ≤4.00 mm with radiation time 268 s). When necessary, the lesions were redilated using the same balloon catheter. All maneuvers requiring handling of radiation (source, catheter and application) were performed by medical physicists and radiation therapists, while all other steps in the procedure were carried out by cardiologists. Quantitative analysis of angiograms was performed before, during and after the intervention. The initial heparin dose was 5000 U, with 2500 U being administered for each additional hour or according to activated coagulation time, which was monitored regularly. Prophylactic abciximab was administered in 11 of the 12 patients. Mean fluoroscopy time was 10.9±5 min (4.8–24.4) and mean procedural time was 50±16 min (33–79).
Angiographic success was defined as <50% final residual stenosis, and clinical success as angiographic success together with absence of clinical complications during hospital stay.
Patients’ initial medication was continued, and dual antiplatelet therapy with aspirin and clopidogrel or ticlopidine was added. All patients have been followed regularly up to the present or until death, and control angiography was scheduled in all patients nine months after the intervention.
Following the recommendations of the Academic Research Consortium (ARC),21 clinical follow-up considered cardiovascular and all-cause mortality, any MI, any new revascularization, angiographic restenosis, and stent thrombosis according to the ARC's new definition.21 The incidence of major adverse cardiac events (MACE), defined as the combined incidence of cardiac death, MI and urgent target vessel revascularization (TVR), was also assessed.
Statistical analysisPatients were analyzed according to continuous and categorical variables. Categorical variables were characterized in terms of absolute and relative frequencies. The central tendency and distribution of continuous variables were estimated by sample mean and standard deviation. Event-free survival was analyzed using Kaplan-Meier curves.
The statistical analysis was performed using SPSS version 19.0.0.2.
ResultsIn all cases there was both clinical and angiographic success. The degree of stenosis decreased from 64.4±16.9 to 25.9±10.3% and minimal luminal diameter increased from 1.0±0.12 to 2.18±0.32 mm. No clinical events were recorded during hospital stay.
At nine-month follow-up, only one patient still had angina; restenosis was detected and the patient subsequently underwent CABG.
Follow-up was achieved in all patients, for a mean of 10.9±2.5 years. During this period, 19 events occurred in seven patients (Tables 3 and 4). Three patients (25%) died, one from cancer at 90 months after the intervention, one at 132 months of sepsis (neither having suffered any cardiac event after brachytherapy), and one (8.3%) from sudden cardiac death at 43 months, a few days after undergoing PCI and implantation of two DES in a different vessel from that treated by brachytherapy.
Outcomes.
| Mean follow-up (years) | 10.9±2.5 |
| Patients with events | n (%) |
| MACE | 2 (16.6) |
| Cardiac death | 1 (8.3) |
| MI | 1 (8.3) |
| Urgent TVR | 0 |
| All-cause death | 3 (25) |
| TVR | 5 (41.6) |
| Any revascularization | 5 (41.6) |
| Definite stent thrombosis | 1 (8.3) |
| Probable stent thrombosis | 1 (8.3) |
| Possible stent thrombosis | 0 |
| Events related to the vessel treated by brachytherapy (n) | |
| Cardiac death | 1 |
| Angiographic restenosis at nine monthsa | 3 |
| Total TVR | 10 |
| Patients with at least one TVR | 5 |
| Total TVR by CABG | 4 |
Description of events.
| Patient no. | Clinical status at nine months | ISR | Revasc | EventMonths FUP | EventMonths FUP | EventMonths FUP | EventMonths FUP | EventMonths FUP | Clinical statusMonths FUP |
| 2 | Asymptomatic | No | Died (cancer)90 months | ||||||
| 3 | Asymptomatic | Yes(occlusion) | TVR(CABG)23 months | Asymptomatic149 months | |||||
| 5 | Asymptomatic | No | NTVR | NTVR | TVR(CABG)21 months | NTVR | NTVR | STEMISTNTVR120 months | Asymptomatic145 months |
| 7 | Asymptomatic | Yes(occlusion) | TVR9 months | TVR(DES)13 months | TVR(DES)19 months | TVR(CABG)35 months | Asymptomatic140 months | ||
| 8 | Asymptomatic | No | TVR9 months | TVR(DES)30 months | NTVR | MSC43 months | Sudden cardiac death43 months | ||
| 9 | Asymptomatic | Angiography not performed | Died (sepsis)132 months | ||||||
| 12 | Angina | Yes | TVR(CABG)14 months | Asymptomatic |
CABG: coronary artery bypass grafting; DES: drug-eluting stent; FUP: follow-up; ISR: in-stent restenosis at nine months; NTVR: non-target vessel revascularization; Revasc: revascularization at nine months; ST: stent thrombosis; STEMI: ST-segment elevation myocardial infarction; TVR: target vessel revascularization.
At 120 months after brachytherapy, one patient (8.3%) suffered ST-segment elevation myocardial infarction (STEMI) due to stent thrombosis in a non-target vessel and underwent successful primary PCI.
Five patients (42%) underwent 15 revascularizations, at least one of the target vessel (a total of nine), mainly in the first two years, including four patients (33.3%) who underwent CABG.
The first TVR occurred on average 12±4.5 months after brachytherapy (9–21 months). Of the five patients who underwent TVR, in three cases this followed scheduled elective angiography that revealed restenosis of the target vessel, two of the patients being asymptomatic.
There was one definite stent thrombosis, in a non-target vessel (8.3%), 120 months after brachytherapy, in an individual who three months previously had undergone drug-eluting balloon angioplasty of a previously implanted DES. One patient (8.3%) had a probable stent thrombosis, identified after sudden cardiac death following revascularization of a non-target vessel 45 months after brachytherapy. No cases of possible stent thrombosis, or of definite or probable stent thrombosis in vessels treated by brachytherapy, were identified.
At 10-year follow-up, the incidence of MACE was 16.6% (two patients): one sudden cardiac death and one non-fatal STEMI. No patient required urgent TVR.
Control coronary angiography to screen for restenosis was performed on average 271.6±50.6 days after brachytherapy in the 11 patients who agreed to undergo the exam. The rate of angiographic restenosis in these patients was 27% (three patients); asymptomatic occlusion was detected in two cases (18%).
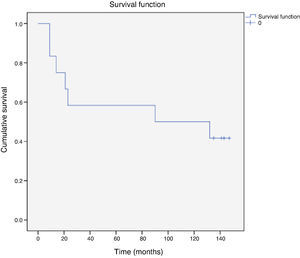
Cardiovascular event-free survival at 10 years was 42% (five patients) (Figure 1).
DiscussionThis article presents the initial and long-term results of the single and limited experience of intracoronary brachytherapy for the treatment of diffuse BMS ISR in Portugal.
The small number of patients means that conclusions cannot be drawn concerning the value of brachytherapy compared to other techniques used at the time to treat ISR. However, the long follow-up affords an opportunity to increase our knowledge of the late effects of intracoronary radiation.
When brachytherapy was introduced in Portugal, the technique was the best option for treating ISR, which had been shown to be the main obstacle to wider use of BMS in certain situations, such as long lesions, recurrent restenosis and in diabetic patients. Various studies demonstrated its short-term safety and efficacy and advantages over pharmacological therapy, balloon dilatation, cutting balloon, rotational atherectomy, laser coronary angioplasty, and stent-in-stent.2–11,22 Concerns about the technique focused on three problems: (1) ‘geographic miss’, by which radiation does not cover the distal and proximal ends of the stent, leading to restenosis; (2) stent thrombosis; and (3) late restenosis of the target vessel treated by brachytherapy, known as ‘late catch-up’. In our experience, the first two problems were solved by using a 40-mm delivery catheter with a pullback technique, and by prescribing dual antiplatelet therapy (thienopyridines and aspirin) for all patients at least until the control angiogram. The long follow-up period may clarify the risk and extent of late restenosis and stent thrombosis in treated segments.
There were no immediate complications, either clinical or vessel-related, in any of the patients, and quantitative angiography showed excellent early results.
The rate of restenosis on control angiography at nine months (27%) in our study is similar to that reported in the literature. In randomized trials of beta radiation for ISR, the mean restenosis rate at 6–9 months was 30% (26%–34%).9–11 A surprising finding was two cases (18%) of occlusive target vessel restenosis, despite dual antiplatelet therapy, in patients who did not suffer acute coronary syndrome, and were in fact asymptomatic. In a review by Waksman et al.23 of 473 patients treated by beta and gamma brachytherapy and who received only one month of dual antiplatelet therapy, late occlusion occurred in 9.1%, and only 7% of these were asymptomatic, while 43% had MI and 50% presented unstable angina. The possibility of late restenosis after brachytherapy for ISR has been demonstrated in studies with longer follow-up (up to five years), frequently necessitating late TVR.16,17
In our study, with a mean 10-year follow-up, the incidence of MACE was 16.6%. This figure is low considering the severity of the patients treated and the long follow-up period. In studies with follow-up of up to one year, the mean rate of MACE was 23% (15%–34%).9–11 In the only published study with a 10-year follow-up (by the Thoraxcenter, Rotterdam20), in 124 patients treated by brachytherapy for ISR, death from any cause was 16% and death or non-fatal MI was 25%. The combination of all-cause death, any MI and any revascularization was observed in 70% of these patients, mainly new TVR (47%) between five months and two years of follow-up. Late catch-up following beta brachytherapy was mainly seen in unstented (de novo) lesions.
Despite concerns regarding increased incidence of acute coronary events following brachytherapy, only one patient (8.3%) in this long follow-up suffered acute coronary syndrome, a complication of previous PCI in another vessel. The only cardiac death was late, and occurred almost immediately after complex PCI in a non-target vessel. It can be fairly confidently assumed that these events cannot be attributed to brachytherapy.
In our center, 42% of the patients (n=5) required multiple (n=9) revascularizations of vessels treated by brachytherapy. This is a high proportion compared to the current efficacy of DES, as demonstrated by trials comparing the two approaches.24–28 However, the complexity of our study population should be borne in mind: 50% with more than one previous restenosis of the treated segment, 67% with multivessel disease, 42% with diabetes and 50% with peripheral arterial disease.
The fact that control angiography was scheduled for nine months may have affected the rate of new TVR observed in our study. The phenomenon of ‘oculostenotic reflex’ has been recognized since the Benestent II study.29 Of the five patients who underwent TVR, four had restenosis on angiography but no angina or positive ischemia test and thus the procedure was not ischemia-driven.
The demonstrated efficacy of DES in treating ISR has reduced the use of brachytherapy for this purpose. In a meta-analysis of randomized studies comparing first-generation DES (sirolimus and paclitaxel) with balloon angioplasty or brachytherapy for treatment of ISR, there was a 65% reduction in TVR and 64% reduction in angiographic restenosis with DES compared to balloon angioplasty or brachytherapy, with no difference in the incidence of death or MI, in a follow-up of 9–12 months.24 In the TAXUS V ISR trial,25 placlitaxel-eluting stents significantly reduced the need for ischemia-driven TVR compared to beta brachytherapy (18.1% vs. 27.5%, p=0.03) in a two-year follow-up. The SISR trial26 compared sirolimus-eluting stents and beta or gamma brachytherapy; at three years, the incidence of TVR was not significantly lower with DES (20.8% vs. 29.6%, p=0.073). At the five-year follow-up of the same study, TVR rates remained similar (24.7% with sirolimus-eluting stents vs. 31.2% with brachytherapy, p=0.179).27 A more recent trial by Wiemer et al.28 comparing beta brachytherapy and sirolimus-eluting stents in a three-year follow-up showed that the TVR rate with brachytherapy at six months (10.4%) (vs. 2.3% with stents, p=0.25) increased to 46.7% (vs. 11.6% with stents, p<0.0001) at three years.
One important point is that of the five patients who underwent TVR, this was by CABG in three, and these patients had no further target vessel-related events in the rest of follow-up. By contrast, the two patients implanted with DES both suffered clinical restenosis, one of whom underwent CABG and the other suffered sudden death following PCI of a non-target vessel. It should however be pointed out that there was no significant occlusive disease in the anterior descending artery in these two patients.
Brachytherapy has recently resurfaced as an option for the treatment of restenosis of DES, but it is rarely used.30
In conclusion, in our limited experience, intracoronary brachytherapy to treat diffuse restenosis of BMS is safe, with acceptable rates of angiographic restenosis at nine months and MACE at 10 years, bearing in mind the characteristics of the population treated. Although the number of TVRs was high, they were required in only five patients, while 41% of patients were event-free at the end of the follow-up period, confirming the long-term safety of the procedure.
LimitationsThe authors acknowledge the following limitations to the present study:
- (1)
It was a retrospective, observational study in a single center, with a small patient sample.
- (2)
There was no control group, the results being interpreted by comparison with those in the literature.
- (3)
The patients included were a highly selected subgroup of considerable clinical and angiographic complexity followed over the course of 10 years, which should be borne in mind when interpreting the rate of clinical events recorded during follow-up.
- (4)
Since this was our center's initial experience with this technique, the results inevitably reflect the learning curve associated with any new procedure.
- (5)
Procedures performed 10 years ago may not reflect current practice, which will have been influenced by changes in therapeutic strategies, available devices, and the evidence base.
- (6)
The control angiography scheduled for all patients, irrespective of evidence of ischemia, may have overestimated the need for TVR.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful to SOCIME Medical, in the person of Dr. Paulo Amaro, for their provision of the material used in beta brachytherapy. They also thank the Francisco Gentil Portuguese Oncology Institute for its assistance in putting together the multidisciplinary team (radiation therapists L. Jorge and M. Roldão, and medical physicists N. Teixeira and P. Ferreira) and for storing the radioactive source, and Bristol Myers Squibb Farmacêutica Lda. for supplying clopidogrel to all patients up to the nine-month control angiogram. Finally we thank all the physicians, nurses, technicians and secretarial staff of the Cardiology Department of Hospital de Santa Cruz, particularly the Cardiac and Vascular Intervention Unit, for their assistance in the brachytherapy procedures.
Please cite this article as: Seabra Gomes R, de Araújo Gonçalves P, Campante Teles R, et al. Avaliação tardia (>10 anos) da braquiterapia intracoronária com radiação beta para reestenose difusa intrastent. Rev Port Cardiol. 2014;33:609–616.