Beta-blockers are recommended after ST-elevation myocardial infarction (STEMI), but their benefit in patients with preserved left ventricular ejection fraction (LVEF) is unclear.
MethodsConsecutive patients discharged in sinus rhythm after STEMI between January 2010 and April 2015 were followed until December 2017. Percutaneous coronary intervention (PCI) was performed in 969 (99.7%, including 112 with rescue PCI) and three (0.3%) received only thrombolytic therapy without rescue PCI.
ResultsOf these 972 patients, mean age 62.6±13.5 years, 212 (21.8%) were women and 835 (85.9%) were prescribed beta-blockers at discharge. Patients who did not receive beta-blockers had more comorbidities than those who did, including chronic obstructive pulmonary disease (14.6% vs. 4.2%), anemia (8.0% vs. 3.7%), and cancer (7.3% vs. 2.8%), and more frequently had inferior STEMI (75.9% vs. 56.0%) and high-grade atrioventricular block (13.1% vs. 5.3%) (all p<0.01). After a mean follow-up of 49.6±24.9 months, beta-blocker treatment at discharge was independently associated with lower mortality (HR 0.61, 95% confidence interval [CI] 0.38-0.96, p=0.03). This effect was present in 192 patients with LVEF ≤40% (HR 0.57, 95% 95% CI 0.34-0.97, p=0.04) but was not clear in 643 patients with LVEF >40% (HR 0.67, 95% 95% CI 0.25-1.76, p=0.42).
ConclusionIn the LVEF >40% group, the results raise reasonable doubts about the real benefit of systematic use of beta-blockers as treatment for these patients. These findings reinforce the need for large randomized clinical trials within this group of patients.
Os betabloqueantes são recomendados após enfarte agudo do miocárdio com elevação do segment ST (STEMI). No entanto, é pouco claro o seu benefício em doentes com fração de ejeção ventricular esquerda (FEVE) preservada.
MétodosDoentes consecutivos com alta hospitalar em ritmo sinusal após STEMI entre janeiro de 2010 e abril de 2015 foram seguidos até dezembro de 2017. A intervenção coronária percutânea (ICP) foi feita em 969 doentes (99,7%, inclusive 112 com ICP de recurso), os restantes 3 (0,3%) receberam apenas terapêutica trombolítica sem ICP de recurso.
ResultadosDos 972 doentes, idade média 62,6 ± 13,5 anos, 212 (21,8%) eram mulheres e 835 (85,9%) estavam a medicados com betabloqueantes no momento da alta hospitalar. Os doentes não medicados com betabloqueantes apresentaram mais comorbilidades do que os tratados com esses fármacos, inclusive doença pulmonar obstrutiva crónica (14,6% versus 4,2%), anemia (8,0% versus 3,7%) e neoplasia (7,3% versus 2,8%) e tiveram mais frequentemente STEMI inferior (75,9% versus 56,0%), bloqueio auriculoventricular de alto grau (13,1% versus 5,3%). Todos os valores corresponderam a p < 0,01. Após um seguimento médio de 49,6 ± 24,9 meses, a terapêutica com betabloqueantes no momento da alta hospitalar associou-se independentemente à mortalidade inferior (hazard ratio [HR] 0,61, intervalo de confiança [IC] 0,38-0,96, p = 0,03). Esse efeito verificou-se em 192 doentes com FEVE ≤ 40% (HR 0,57, IC 0,34-0,97, p = 0,04).No entanto, não foi clara essa vantagem em 643 doentes com FEVE > 40% (HR 0,67, IC 0,25-1,76, p = 0,42).
ConclusãoNo grupo com FEVE > 40%, os resultados levantam dúvidas sobre o benefício real da administração sistemática de betabloqueantes como forma de tratamento para esses doentes. Esses achados reforçam a necessidade de grandes ensaios clínicos aleatorizados sobre esse grupo de doentes.
The benefit of beta-blockers is undisputed in patients with heart failure (HF) or left ventricular systolic dysfunction,1,2 and they are recommended in the guidelines for patients after ST-segment elevation myocardial infarction (STEMI) in order to reduce hospitalizations and mortality. The European guidelines3 confer a class IIa recommendation in patients without HF who present normal LVEF, while in the American guidelines4 they have a class I indication for all patients regardless of HF or LVEF. Most trials assessing the effect of beta-blockers after STEMI were carried out several decades ago.5 In the reperfusion era, the benefit of beta-blockers is less clear and seems to be focused on high-risk patients6 such as those with depressed left ventricular ejection fraction (LVEF),7 anterior infarction8 or multivessel disease.9 In cases of preserved LVEF, the evidence for the benefit of beta-blockers is inconclusive.5–13
The aim of our study was to assess the long-term benefit of beta-blockers in a contemporary population of patients discharged in sinus rhythm after STEMI and to study the influence of LVEF on this benefit.
MethodsOur data come from the Description of Acute Myocardial Infarction: Management, New Therapies and Evolution (DIAMANTE) registry. The study's methodology has been previously published.14–17 In this paper all patients are included who were 18 years of age or older discharged alive in sinus rhythm after a STEMI18 between January 2010 and April 2015. Exclusion criteria were as follows: presentation more than 24 hours after symptom onset or no reperfusion therapy; out-of-hospital cardiac arrest; need for endotracheal intubation prior to hospital arrival; and non-obstructive coronary artery disease and no evidence of cardiac emboli as the cause of the STEMI. Patients were divided according to the use of beta-blockers at discharge. LVEF was measured by echocardiography and classified as ≤40% or >40%. The primary endpoint was all-cause death during follow-up. Other endpoints assessed included major adverse cardiac events (defined as a composite of all-cause death, reinfarction, vascular complication and hospitalization due to HF) at 30 days after discharge, stroke, and atrial fibrillation during follow-up.
The study complies with the Declaration of Helsinki and was approved by the Ethics Committee of our institution.
Statistical analysisContinuous variables are presented as means ± standard deviation) and categorical variables are presented as frequencies and percentages. Comparisons between groups were made using the Student's t test, or the nonparametric Mann-Whitney U test when appropriate, for continuous variables and the chi-square test for categorical variables. Odds ratios (OR) and their 95% confidence intervals were calculated by logistic regression modeling to identify predictors of beta-blockers use at discharge. Adjusted hazard ratios (HR) and their 95% confidence intervals were calculated for the primary and secondary outcome measurements in all patients and also according to LVEF (≤40% or >40%). Cumulative incidences of clinical event rates were estimated by the Kaplan-Meier method and Cox regression analysis. Statistical analysis was performed using IBM SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
ResultsA total of 972 patients were included, with a mean age of 62.6±13.5 years, 212 (21.8%) of whom were women. Percutaneous coronary intervention (PCI) was performed in 969 (99.7%, including 112 with rescue PCI) and three (0.3%) received only thrombolytic therapy without rescue PCI. Beta-blockers were prescribed at discharge in 835 patients (85.9%). Baseline characteristics according to beta-blocker prescription are described in Table 1. Patients who did not receive beta-blockers had more comorbidities than those treated with beta-blockers, including chronic obstructive pulmonary disease, anemia, and cancer, and more frequently had inferior STEMI and high-grade atrioventricular block. The independent predictors of beta-blocker prescription at discharge by multivariate analysis are shown in Table 2.
Population characteristics according to beta-blocker prescription at discharge.
| All (n=972) | Beta-blocker (n=835) | No beta-blocker (n=137) | p | |
|---|---|---|---|---|
| Age, years | 62.6±13.5 | 62.8±13.7 | 62.5±13.5 | 0.81 |
| Female | 212 (21.8%) | 176 (21.1%) | 36 (26.3%) | 0.11 |
| Hypertension | 504 (51.9%) | 440 (52.7%) | 64 (46.7%) | 0.11 |
| Diabetes | 197 (20.3%) | 162 (19.4%) | 35 (25.5%) | 0.06 |
| Dyslipidemia | 441 (45.4%) | 382 (45.7%) | 59 (43.1%) | 0.31 |
| Active smoking | 463 (47.6%) | 397 (47.5%) | 66 (48.2%) | 0.48 |
| BMI, kg/m2 | 27.7±4.3 | 27.8±4.3 | 26.9±4.3 | 0.97 |
| COPD | 55 (5.7%) | 35 (4.2%) | 20 (14.6%) | <0.001 |
| Previous AF | 9 (0.9%) | 7 (0.8%) | 2 (1.5%) | 0.37 |
| Chronic HF | 40 (4.1%) | 35 (4.2%) | 5 (3.6%) | 0.5 |
| Previous cardiac surgery | 12 (1.3%) | 11 (1.3%) | 1 (0.7%) | 0.48 |
| CKD | 59 6.1%) | 48 (5.7%) | 11 (8.0%) | 0.2 |
| PAD | 35 (3.6%) | 25 (3.0%) | 10 (7.3%) | 0.02 |
| Anemia | 32 (3.3%) | 21 (3.7%) | 11 (8.0%) | 0.003 |
| Active cancer | 33 (3.4%) | 23 (2.8%) | 10 (7.3%) | 0.01 |
| Infarct location | ||||
| Anterior | 398 (40.9%) | 366 (43.8%) | 32 (23.4%) | <0.001 |
| Inferior, lateral or posterior | 572 (58.8%) | 468 (56.0%) | 104 (75.9%) | |
| LBBB | 2 (0.2%) | 1 (0.1%) | 1 (0.7%) | |
| Right ventricular infarction | 71 (7.3%) | 52 (6.2%) | 19 (13.9%) | 0.03 |
| High-grade AVB at admission | 62 (6.4%) | 44 (5.3%) | 18 (13.1%) | 0.001 |
| LVEF (%) | 47.2±10.9 | 46.7±10.9 | 50.7±10.6 | <0.001 |
| LVEF ≤40% | 208 (21.4%) | 192 (23.0%) | 16 (11.7%) | 0.01 |
| VF | 70 (7.2%) | 62 (7.4%) | 8 (5.8%) | 0.32 |
| Killip class ≥II | 157 (16.2%) | 132 (15.8%) | 25 (18.2%) | 0.27 |
| Hospital stay (days) | 6.76±14.40 | 6.73±15.01 | 6.99±10.4 | 0.85 |
| Complications | ||||
| Cardiogenic shock | 103 (10.6%) | 82 (9.8%) | 21 (15.3%) | 0.04 |
| AF during admission | 60 (6.2%) | 47 (5.6%) | 13 (9.5%) | 0.07 |
| Ventricular arrhythmias post-STEMI | 18 (1.9%) | 14 (1.7%) | 4 (2.9%) | 0.24 |
| Major bleeding | 31 (3.2%) | 22 (2.6%) | 9 (6.6%) | 0.02 |
| Cardiac tamponade | 9 (0.9%) | 5 (0.6%) | 4 (2.9%) | 0.03 |
| AKI | 71 (7.3%) | 62 (7.4%) | 9 (6.6%) | 0.44 |
| Stroke | 6 (0.6%) | 4 (0.5%) | 2 (1.5%) | 0.005 |
| Treatment | ||||
| Fibrinolysis | 115 (11.8%) | 104 (12.5%) | 11 (8.0%) | 0.08 |
| Radial access for angiography | 734 (75.5%) | 640 (76.6%) | 94 (68.6%) | 0.03 |
| DES | 597 (61.4%) | 527 (63.1%) | 70 (51.1%) | 0.001 |
| Complete revascularization | 744 (76.5%) | 637 (76.3%) | 107 (78.1%) | 0.49 |
| ACE inhibitors at discharge | 830 (85.4%) | 735 (88.0%) | 95 (69.3%) | <0.001 |
ACE: angiotensin-converting enzyme; AF: atrial fibrillation; AKI: acute kidney injury; AVB: atrioventricular block; BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DES: drug-eluting stent; HF: heart failure; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; PAD: peripheral arterial disease; STEMI: ST-elevation myocardial infarction; VF: ventricular fibrillation.
Independent predictors of beta-blocker prescription at discharge.
| OR | 95% CI | p | |
|---|---|---|---|
| Hypertension | 1.58 | 1.04-2.42 | 0.03 |
| PAD | 0.39 | 0.17-0.89 | 0.03 |
| COPD | 0.23 | 0.12-0.45 | <0.001 |
| High-grade AVB | 0.50 | 0.26-0.95 | 0.04 |
| HF/cardiogenic shock | 0.48 | 0.26-0.90 | 0.02 |
| LVEF | 0.97 | 0.94-0.99 | 0.003 |
AVB: atrioventricular block; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HF: heart failure; LVEF: left ventricular ejection fraction; OR: odds ratio; PAD: peripheral arterial disease.
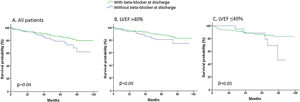
During a mean follow-up of 49.6±24.9 months, 114 patients died (11.7%). Long-term mortality was lower in those who received beta-blockers at discharge than in those who did not. Beta-blocker treatment at discharge was independently associated with lower mortality, but this effect was mainly present in 192 patients with LVEF ≤40% and was not clear in 643 patients with LVEF >40% (Table 3). Survival curves according to the use of beta-blockers at discharge are shown in Figure 1.
Independent predictors of long-term mortality.
| HR | 95% CI | p | |
|---|---|---|---|
| All patients | |||
| Age | 1.06 | 1.04-1.08 | <0.001 |
| Creatinine | 1.48 | 1.25-1.75 | <0.001 |
| Killip class ≥II | 1.55 | 1.03-2.32 | 0.03 |
| Beta-blocker at discharge | 0.61 | 0.38-0.96 | 0.03 |
| LVEF ≤40% | |||
| Age | 1.07 | 1.05-1.09 | <0.001 |
| Creatinine | 1.46 | 1.18-1.81 | 0.001 |
| Killip ≥II | 1.37 | 0.80-2.34 | 0.25 |
| Beta-blocker at discharge | 0.57 | 0.34-0.97 | 0.04 |
| LVEF >40% | |||
| Age | 1.04 | 1.01-1.07 | 0.02 |
| Creatinine | 1.42 | 1.08-1.88 | 0.01 |
| Killip class ≥II | 1.93 | 0.91-4.11 | 0.09 |
| Beta-blocker at discharge | 0.67 | 0.25-1.76 | 0.42 |
CI: confidence interval; HR: hazard ratio; LVEF: left ventricular ejection fraction.
In our contemporary cohort of STEMI patients treated with urgent reperfusion therapy and discharged in sinus rhythm, the use of beta-blockers at discharge was associated with better long-term clinical outcomes, particularly in patients with LVEF ≤40%.
The beneficial effect of beta-blocker therapy in STEMI patients is related to reduction in myocardial oxygen demand (by decreasing heart rate, systemic blood pressure, and myocardial contractility) and to increased diastolic perfusion of the ischemic territory, limiting infarct size.7 In the prefibrinolytic era, beta-blocker therapy after STEMI was associated with lower mortality and reinfarction.19 However, in the reperfusion era this beneficial effect is less pronounced.20 In the COMMIT trial,21 metoprolol (intravenous followed by oral administration) had no effect on mortality but reduced the risk of recurrent myocardial infarction at 28 days, in a population without primary PCI and fibrinolysis administered in only 55%. In the last decade the management of patients with STEMI has changed dramatically, including widespread use of primary PCI, efforts to shorten door-to-balloon time, technical and technological improvements in PCI, and widespread use of evidence-based medications such as statins, newer antiplatelet agents, and drugs that inhibit the renin-angiotensin-aldosterone system.7 As a result of these advances, morbidity and mortality after acute STEMI have improved markedly.22,23 Consequently, in some cases, the benefit of beta-blockers may be diluted.
Our data suggest that after STEMI, beta-blocker therapy is associated with better long-term prognosis, particularly with LVEF ≤40%, and this finding is consistent with previous studies.5–7,9 Some studies have demonstrated a benefit of beta-blockers in patients with preserved LVEF.10,11,13 The relatively small sample size of some patient subgroups and the limitations inherent to observational studies may explain these differences.
In a recent study by Dondo et al., 24 the largest analysis to date (comprising >180000 cases) of the effect of beta-blockers on mortality after acute myocardial infarction without HF or depressed LVEF, the use of these drugs was not associated with a lower risk of death. Prior to that study, a meta-analysis of 10 observational studies across 40873 patients suggested a lack of evidence to support the routine use of beta-blockers in all patients with myocardial infarction treated with PCI, but the effect was restricted to reduced LVEF, non-STEMI, and patients with low use of secondary prevention medications.25
The guidelines differ in their recommendations regarding the use of beta-blockers after STEMI. The European guidelines3 confer a class IIa recommendation, level of evidence B, in patients without HF who present normal LVEF, while in the American guidelines4 they have a class I indication for all patients regardless of HF or LVEF. Many patients are prescribed beta-blockers indefinitely after STEMI regardless of LVEF.26 Dondo et al.24 suggest, and we agree, that this practice is probably based on clinical uncertainty.
As beta-blockers are not free of side effects (mild to severe hypotension, bradycardia, dizziness, depression, metabolic disorders, and drug allergy20), and a increasing number of medications is associated with reduced adherence,27 beta-blockers probably should not be mandatory in the discharge treatment of patients with STEMI and LVEF >40%. Randomized trials are needed to resolve questions in this regard.
LimitationsObservational studies are vulnerable to selection bias and unidentified confounding factors, so our study has some limitations that must be recognized. The use of beta-blockers in our study was determined by medication at discharge or in-hospital prescription, while adherence was less clear and we did not have data regarding beta-blocker use after discharge. We did not perform propensity-score matching because there were no significant differences between the groups in age, gender or main risk factors, although we recognize that there may still be risk differences in other variables and this may imply additional selection biases. Also, the sample size of patients without beta-blockers was relatively small. Nevertheless, our findings are consistent with other non-randomized studies.
ConclusionIn patients discharged in sinus rhythm after STEMI treated with PCI, beta-blocker therapy was independently associated with lower mortality in patients with LVEF ≤40%, but the benefit was doubtful in those with LVEF >40%. These findings reinforce the need for large randomized clinical trials in this group of patients.
Conflicts of interestThe authors have no conflicts of interest to declare.