The association between hypocalcemia and heart failure is rare. There are few reported cases in the literature of this association, which is termed hypocalcemic cardiomyopathy.
We report the case of a 61-year-old woman with no relevant medical history, admitted for progressively worsening exertional dyspnea, orthopnea and edema of the lower limbs over a period of one month. Physical examination showed diffuse muscle spasms, with no signs of latent tetany.
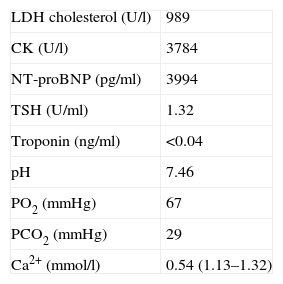
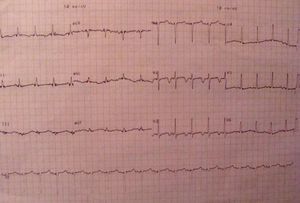
Further investigation revealed ionized calcium 0.54 mmol/l (normal 1.12–1.30), phosphorus 9.8 mg/dl, parathyroid hormone <2.5 pg/ml and CK >3000 U/l, with normal thyroid function. The electrocardiogram showed long QT interval and a pattern of left ventricular overload, and myocardial biomarkers were negative. The echocardiogram revealed regional wall motion abnormalities, coronary angiography was normal and a cranial CT scan detected calcification of basal ganglia and white matter.
She started diuretic and calcium replacement therapy which resulted in complete clinical recovery, with no need for heart failure therapy after normalization of serum calcium.
A associação entre hipocalcemia e insuficiência cardíaca é rara. Na literatura existem poucos casos descritos com esta associação, tendo-se estabelecido a entidade miocardiopatia hipocalcemica.
Relata-se o caso de uma mulher, 61 anos, sem antecedentes médicos relevantes. Internada por um quadro com um mês de evolução de dispneia de esforço, ortopneia e edema dos membros inferiores de agravamento progressivo. À observação apresentava espasmos musculares difusos, sem sinais de tetania latente.
Da investigação complementar destacavam-se cálcio ionizado 0,54 mmol/l (1,12-1,30), fósforo 9,8 mg/dl, hormona paratiroideia <2,5 pg/ml e CK total >3000 U/l, com função tiroideia normal. O electrocardiograma revelava prolongamento do intervalo QT e padrão de sobrecarga do ventrículo esquerdo e os marcadores de necrose miocárdica eram negativos. O ecocardiograma demonstrava alterações segmentares da contractilidade miocárdica, a coronariografia era normal e na TC-CE identificavam-se calcificações dos núcleos basais e substância branca.
Iniciou terapêutica diurética e de reposição do cálcio com remissão completa da insuficiência cardíaca, sem necessidade de terapêutica específica para a mesma após normalização da calcemia.
The clinical syndrome of heart failure (HF) results from congenital or acquired alterations to cardiac structure and/or function that are manifested by an imbalance between cardiac output and tissue oxygen requirements.1,2
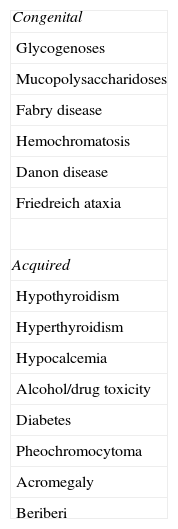
Among the different etiologies of HF are the cardiomyopathies, which are classified according to morphological type as dilated, restrictive and hypertrophic. Some forms of dilated cardiomyopathy, due to metabolic or toxic causes, are reversible (Table 1).3
Calcium plays an essential role in myocardial metabolism, and hypocalcemia reduces myocardial contractility. However, HF of this etiology is rare, with few cases reported in the literature,4 but in most of these cases, correction of hypocalcemia led to resolution of HF.
Only one case has been reported in Portugal of dilated cardiomyopathy associated with post-surgical hypothyroidism, in which hypocalcemia, also in the context of post-surgical hypoparathyroidism, was the factor triggering decompensation of HF.5
Case reportWe report the case of a 61-year-old woman from Brazil, where she had been a mathematics teacher, resident for around a year in Portugal, where she worked as a cleaner. She had a history of surgery for bilateral pseudophakia over 15 years previously; she reported no other previous conditions, relevant family history or cardiovascular risk factors, was taking no medication, and did not drink or smoke.
She had been asymptomatic until three months before admission, when she began to experience worsening exertional dyspnea, associated with orthopnea in the three days before admission. She reported no paroxysmal nocturnal dyspnea, chest pain, palpitations, syncope, cough, expectoration or fever throughout this period.
Due to symptoms on minimal exertion she went to the emergency department, where examination showed mental confusion, psychomotor slowing, depressed facial expression, blood pressure 97/59 mmHg, rhythmic heart rate 79 bpm, respiratory rate 28 cpm, thinning of the outer third of the eyebrows, limb tremor and muscle spasms, but no Chvostek or Trousseau sign. Crackling rales were audible in the lower half of both lung fields, as well as an S3 gallop and a grade I/VI systolic murmur more clearly audible in the mitral area. The rest of the physical exam was normal.
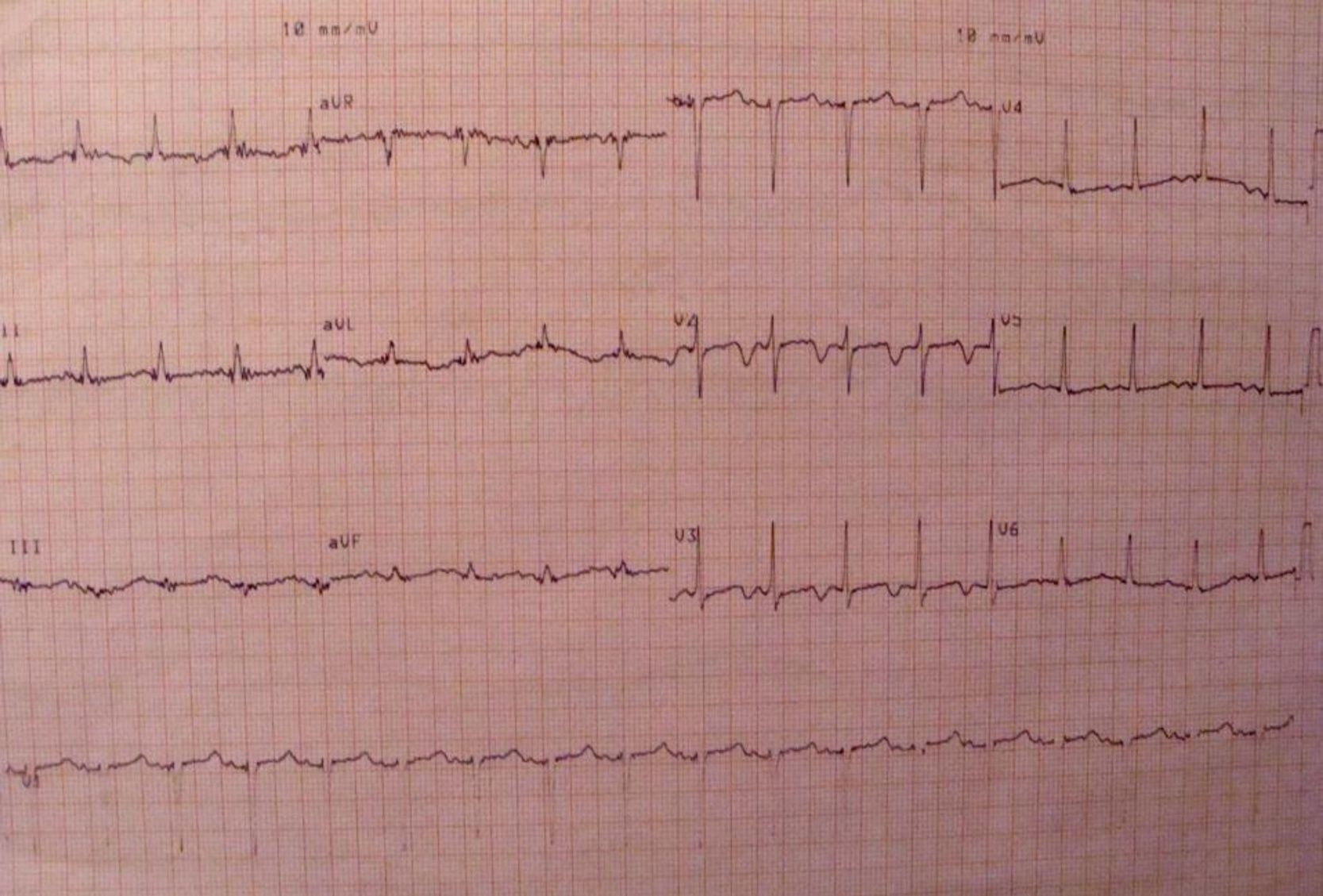
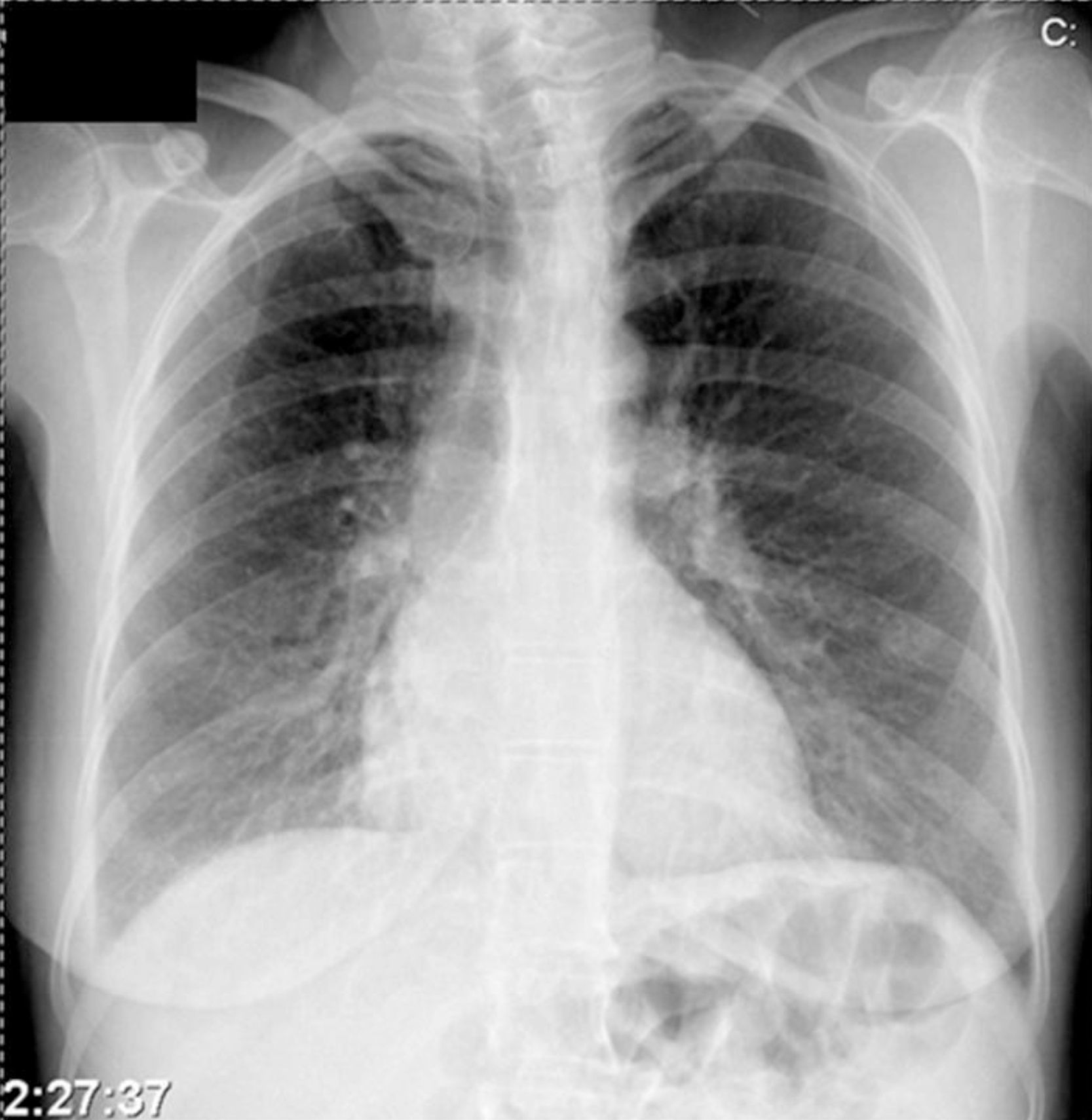
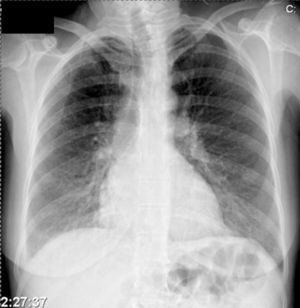
Further diagnostic tests revealed normal myocardial necrosis markers, elevated BNP, rhabdomyolysis, severe hypocalcemia and type 1 respiratory failure (Table 2). The electrocardiogram (ECG) (Figure 1) showed long QT interval (QTc 0.53 s) and T-wave inversion in V2–V4 and DI. The posteroanterior chest X-ray (Figure 2) revealed interstitial infiltration in the lower third of both lung fields, suggestive of edema.
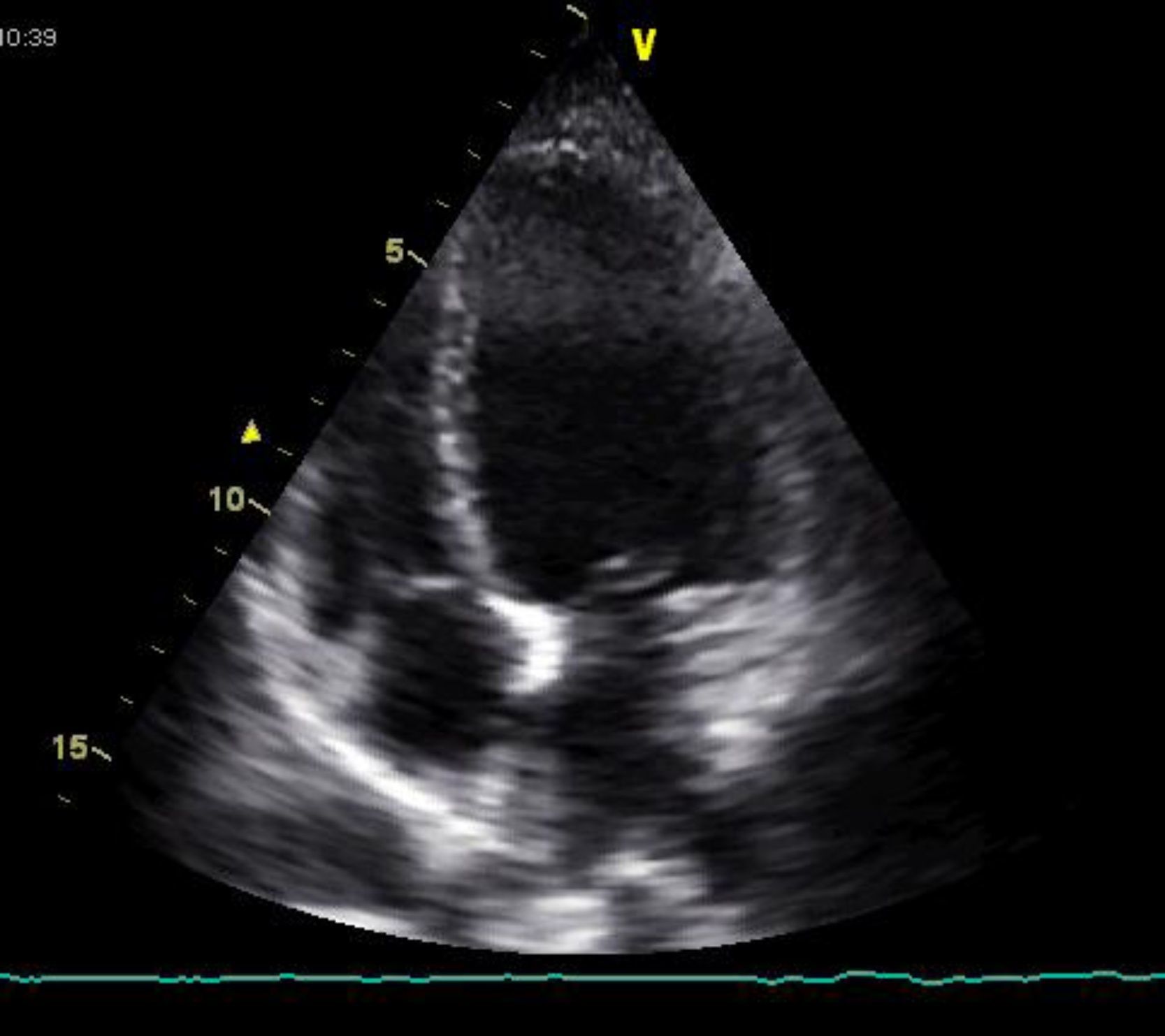
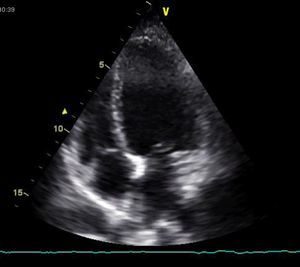
The bedside echocardiogram in the emergency department showed left ventricular dilatation (60/44 mm) with diffuse hypocontractility, more marked in the apex, moderately impaired global systolic function (fractional shortening 26%), but no valvular abnormalities or pericardial effusion (Figure 3). A high-resolution thoracic computed tomography (CT) scan showed a low probability of pulmonary embolism. Serological tests for infectious agents commonly found in myocarditis and Chagas disease were negative.
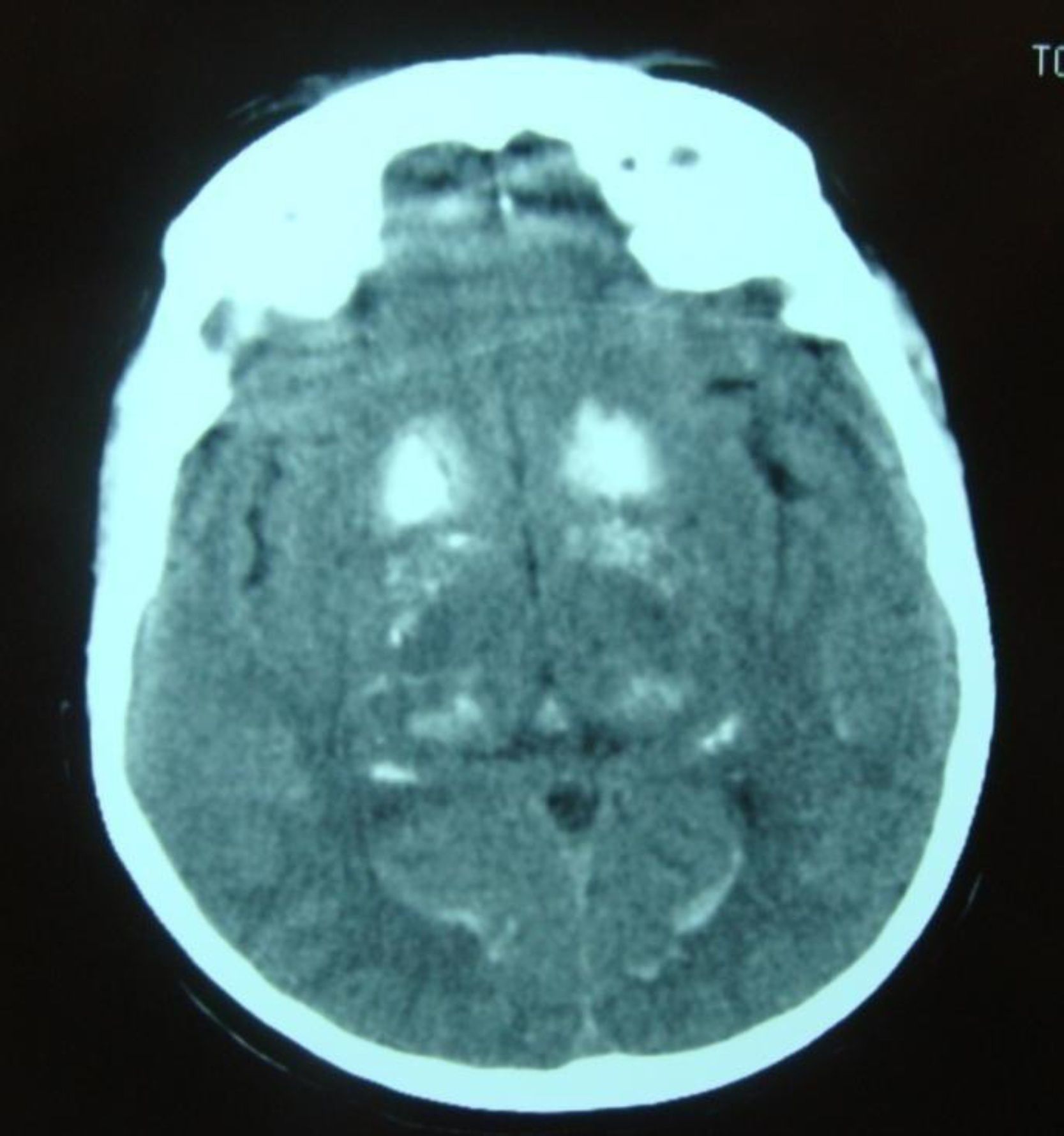
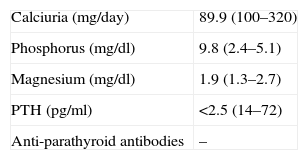
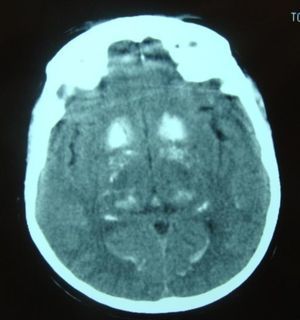
Symptomatic treatment for HF was started with diuretics, angiotensin-converting enzyme (ACE) inhibitors and nitrates, but there was little symptomatic relief and the hypocalcemia was investigated further. Hypocalciuria and hyperphosphatemia were also detected due to reduced parathyroid hormone levels (Table 3). Investigation of the etiology of hypoparathyroidism ruled out cancer, infiltration, and polyglandular and autoimmune syndromes, and a diagnosis of idiopathic hypoparathyroidism was made. Renal ultrasound showed no alterations and a cranial CT scan (Figure 4) detected extensive supratentorial calcification, more evident in the basal ganglia, suggesting typical chronic hypoparathyroidism.
With a provisional diagnosis of HF of unknown etiology and hypocalcemia secondary to idiopathic hypoparathyroidism, the patient continued the above medication, to which were added intravenous calcium carbonate, a phosphate-binding agent (sevelamer) and vitamin D. Progressive normalization of calcium–phosphate metabolism was observed, accompanied by complete reversal of HF symptoms and normalization of ECG and echocardiographic findings (left ventricular size 50/32 mm, no wall motion abnormalities and fractional shortening 36%). Therapy for hypoparathyroidism was maintained but diuretics, nitrates and ACE inhibitors were discontinued. The patient remained asymptomatic.
Cardiac catheterization revealed no significant coronary lesions and normal left ventricular function (ejection fraction 64%).
The association between correction of hypocalcemia and disappearance of HF symptoms, in the absence of an alternative etiology for the latter, confirmed that hypoparathyroidism and resulting hypocalcemia were the cause of her HF.
DiscussionRegulation of serum calcium levels depends mainly on parathyroid hormone, in the short term by calcium reabsorption by bone or kidneys (distal convoluted tubule), and by long-term adaptation, by stimulating renal production of vitamin D.6
Calcium is essential to cardiac automatism and myocardial excitation–contraction coupling, and is thus vital for normal cardiac function. The entry of calcium into myocardial fibers induces release of calcium from the sarcoplasmic reticulum, raising the concentration of intracellular calcium, which binds to troponin C, a process which governs actin-myosin interaction and hence muscle contraction.7,8
The clinical manifestations of acute hypocalcemia are basically due to increased neuromuscular irritability, resulting in parasthesias and limb tremor, rhabdomyolysis, cramps and carpopedal spasm. Latent tetany is often revealed by Chvostek and Trousseau signs. Hypocalcemia can also have neuropsychiatric manifestations including irritability and depression,9,10 which may explain the psychomotor slowing and mental confusion in our patient, who also presented thinning of the outer third of the eyebrows, a typical sign of hypothyroidism, which she did not have. This sign may have been due to local autoimmune phenomena.
In chronic forms of hypocalcemia, manifestations include cataracts, dental dysmorphisms and extrapyramidal symptoms due to calcification of basal ganglia.9,10
Severe hypocalcemia may be accompanied by various cardiovascular manifestations including long QT interval on the ECG, vasodilation with severe hypotension, and congestive HF, all of which are reversible following correction of the ionic imbalance.11,12 This imbalance can result in impairment of myocardial function and hence HF that is usually refractory to conventional treatment, although it is reversible with calcium replacement therapy,10 as seen in our patient. It should also be noted that hypocalcemia reduces natriuresis, which may also contribute to HF.4,13
There are some descriptions in the literature of this rare and reversible cause of HF, which has been termed hypocalcemic cardiomyopathy.4,5,10,13–28 The first report was by Rose in 1943, who suggested “the possibility of some relationship between chronic parathyroid insufficiency and myocardial damage”.29 Transient left ventricular dysfunction has also been documented following blood transfusions with citrate (a calcium-binding agent).18 The common factor in these descriptions is HF in patients with severe hypocalcemia, partially or completely resolved following correction of the ionic imbalance. Various causes of hypocalcemia were reported, including iatrogenic4,24 and autoimmune13,25 hypoparathyroidism and rickets.18,20,28 In some cases coronary insufficiency was not formally excluded by angiography,4,22,25 but in most cases the association with HF was strong.
In the case presented, the fact that the patient received combined therapy for HF and hypocalcemia makes it difficult to determine the precise effect of each treatment on the symptomatic improvement observed. However, in some cases in the literature there was no response to conventional therapy, but there was an unequivocal association between the introduction or discontinuation of calcium replacement therapy and improvement or worsening of HF, respectively.26,27
Although in this case, as in most others, there was normalization of ventricular systolic function, this is not always the case. There is no explanation for this in the literature, but it has been suggested that prolonged exposure of the myocardium to severe hypocalcemia may lead to structural damage, as seen in autopsies of cows that died from hypocalcemic cardiomyopathy, which detected widespread microscopic foci of myocardial necrosis.10,30 Myocardial biopsy in one patient revealed dilated sarcoplasmic reticulum and size variability in the mitochondria, which may have been secondary to metabolic abnormalities.13
Another interesting point is the appearance of acute symptoms of hypocalcemia, including HF, in a patient with signs of chronic disease like calcification of basal ganglia. Decompensation frequently occurs in situations of increased calcium demand such as alkalosis, as seen in our patient, and can perpetuate and worsen hypocalcemia.9 Rhabdomyolysis can be both cause and consequence of hypocalcemia, by altering membrane electrical activity, increasing the electrical excitability of muscle fibers and resulting in tremors, perpetuating the primary abnormality in a vicious cycle.29,31 The duration and severity of hypocalcemia are thus clearly important in triggering cardiac decompensation.22
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Aguiar P, et al. Miocardiopatia hipocalcémica. Rev Port Cardiol. 2013. doi:10.1016/j.repc.2012.08.008.