Non-melanoma skin cancer is the most prevalent malignancy in fair-skinned people and its incidence is increasing. Recently, studies have suggested that antihypertensive drugs may increase the risk of these tumors, particularly hydrochlorothiazide, due to its photosensitizing properties. The Portuguese National Authority for Medicines and Health Products, INFARMED, has issued an alert to healthcare professionals concerning the increased risk of non-melanoma skin cancer in patients exposed to cumulative doses of this drug. However, study results have been heterogeneous and sometimes conflicting. The high incidence of non-melanoma skin cancer and the large number of patients under chronic hydrochlorothiazide therapy may thus have important public health consequences. In this article, the authors review the published evidence and conclude that there may be an association between hydrochlorothiazide use and the risk of non-melanoma skin cancer, but also point out some limitations of the studies in the literature. It is important to promote preventive strategies against sun exposure, regular skin examinations, and individual assessment of the benefits of hydrochlorothiazide use, particularly in patients with previous skin cancer.
O cancro cutâneo não-melanoma é a neoplasia mais prevalente na população caucasiana e a sua incidência está a crescer. Nos últimos anos um conjunto de estudos sugeriram que a terapêutica anti-hipertensora poderá aumentar o risco destes tumores, salientando-se a hidroclorotiazida, pelas suas propriedades fotossensibilizadoras. A crescente evidência disponível levou o INFARMED - Autoridade Nacional do Medicamento e Produtos de Saúde - a realizar um alerta dirigido aos profissionais de saúde para o risco de cancro cutâneo não-melanoma com a exposição dos doentes a doses cumulativas deste fármaco. Contudo, os resultados dos estudos têm-se revelado heterogéneos e, por vezes, contraditórios. A elevada incidência de cancro cutâneo não-melanoma e o grande número de indivíduos a cumprir terapêutica crónica com hidroclorotiazida poderá ter importantes repercussões em termos de saúde pública. Neste artigo, os autores propuseram-se a reunir e analisar a evidência científica disponível, tendo concluído uma possível associação entre a toma de hidroclorotiazida e o risco de cancro cutâneo não-melanoma, não esquecendo, no entanto, algumas limitações dos trabalhos existentes na literatura. O reforço de medidas educacionais para a adoção de estratégias preventivas face à exposição solar, a monitorização regular da pele e avaliação individual do benefício da terapêutica com hidroclorotiazida são importantes, em particular na presença de antecedentes pessoais de tumores de pele.
Non-melanoma skin cancer (NMSC) is the term used for a group of skin cancers that includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). NMSC is the most common type of skin cancer affecting white populations, and its incidence is increasing in Europe and North America.1–3 Portugal is no exception, and the number of NMSC cases diagnosed in this country is rising.4,5
BCC and SCC are locally invasive skin cancers which often affect mask areas of the face, such as the eyelids, and require complex and highly specialized treatment. Despite its low metastatic potential, NMSC is associated with significant morbidity, mainly due to its high recurrence rate.6
The increasing incidence of NMSC poses major challenges for healthcare systems, which are required to adapt their budgets to meet the significantly higher demand for resources for diagnosis, treatment and monitoring of these cancers.7
Although BCC and SCC are distinct clinical entities, they share a common set of risk factors, including exposure to ultraviolet (UV) radiation, mostly chronic and cumulative for SCC and intense and intermittent for BCC, a low Fitzpatrick skin type score (1–2), outdoor occupations and/or hobbies, exposure to artificial ultraviolet (UV) light for cosmetic or therapeutic purposes, and immunosuppressant drug therapy, as shown by the high incidence of these tumors in patients with solid organ transplants.8,9
Over the past decade, efforts to identify additional risk factors have focused on a group of drugs with photosensitizing properties, including antihypertensive agents. However, these studies have generated varying and sometimes conflicting results, which limit the generalizability of their conclusions.
Some studies have recently suggested a strong association between hydrochlorothiazide (HCTZ) therapy and the risk of NMSC.10,11 The available evidence led the Portuguese National Authority of Medicines and Health Products (INFARMED) to issue a warning on the risk of NMSC in patients exposed to cumulative doses of this drug.12
For this review, the authors collected and analyzed the available evidence on the association between HCTZ therapy and the risk of NMSC.
Hydrochlorothiazide and risk of non-melanoma skin cancerIn Portugal, the estimated prevalence of hypertension in adults is 42.2%. When considering only the elderly population (aged 65 years or older), the figure is significantly higher, estimated at 74.9%.13
The 2018 European Society of Cardiology guidelines on hypertension include thiazide diuretics as one of five drug classes recommended for initial therapy and emphasize their efficacy in lowering blood pressure and thus reducing cardiovascular events and mortality.14
Recently, Pinto et al. identified thiazide diuretics, in their study mainly hydrochlorothiazide (HCTZ), as the second most commonly chosen drug class for initial antihypertensive therapy in primary care, surpassed only by angiotensin-converting enzyme inhibitors (ACEIs).15 Similarly, the VALSIM study showed that diuretics were the most commonly used antihypertensive drug class at this level of care.16
Hypertension is mostly diagnosed, and therapy is initiated, in middle-aged adults, who often require long-term and sometimes chronic therapy. The increase in life expectancy observed in recent decades means that a large number of patients are exposed to HCTZ for many years.
HCTZ has proven photosensitizing properties, and may work in synergy with UV radiation in the process of skin carcinogenesis.17 Photosensitivity is defined as an abnormal or exaggerated skin response to exposure to UV radiation or visible light.18 HCTZ appears to act as a chromophore which, after absorbing UVA radiation, transfers energy to adjacent pyrimidine nucleotides, inducing the formation of dimers with mutagenic potential.17
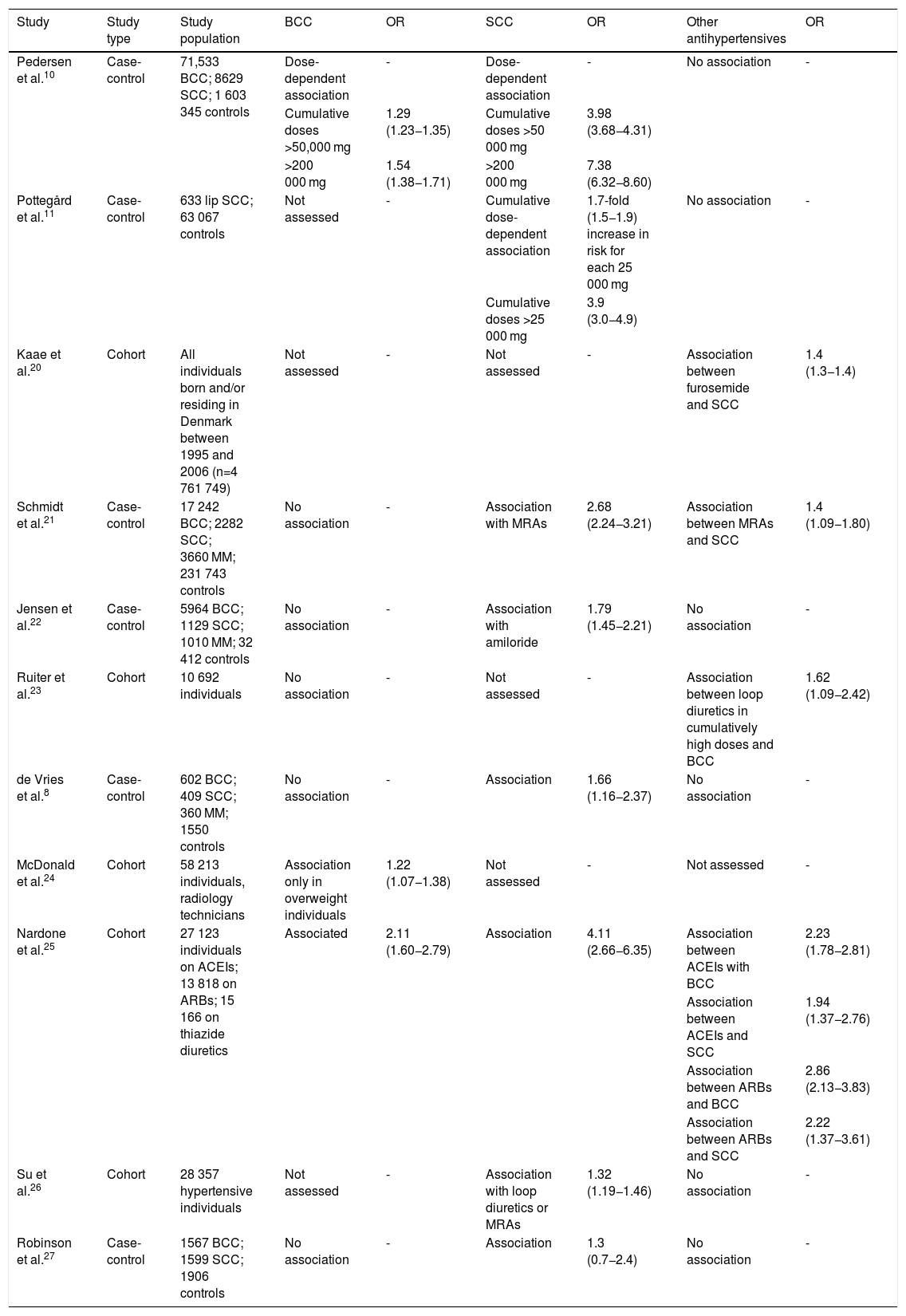
Over the past decade, several studies have been conducted to analyze the association between HCTZ and the risk of NMSC. Table 1 shows some of the elements of their methodology and their main findings.
Main findings of studies analyzing the association of hydrochlorothiazide therapy with the risk of skin cancer.
| Study | Study type | Study population | BCC | OR | SCC | OR | Other antihypertensives | OR |
|---|---|---|---|---|---|---|---|---|
| Pedersen et al.10 | Case-control | 71,533 BCC; 8629 SCC; 1 603 345 controls | Dose-dependent association | - | Dose-dependent association | - | No association | - |
| Cumulative doses >50,000 mg | 1.29 (1.23−1.35) | Cumulative doses >50 000 mg | 3.98 (3.68−4.31) | |||||
| >200 000 mg | 1.54 (1.38−1.71) | >200 000 mg | 7.38 (6.32−8.60) | |||||
| Pottegård et al.11 | Case-control | 633 lip SCC; 63 067 controls | Not assessed | - | Cumulative dose-dependent association | 1.7-fold (1.5−1.9) increase in risk for each 25 000 mg | No association | - |
| Cumulative doses >25 000 mg | 3.9 (3.0−4.9) | |||||||
| Kaae et al.20 | Cohort | All individuals born and/or residing in Denmark between 1995 and 2006 (n=4 761 749) | Not assessed | - | Not assessed | - | Association between furosemide and SCC | 1.4 (1.3−1.4) |
| Schmidt et al.21 | Case-control | 17 242 BCC; 2282 SCC; 3660 MM; 231 743 controls | No association | - | Association with MRAs | 2.68 (2.24−3.21) | Association between MRAs and SCC | 1.4 (1.09−1.80) |
| Jensen et al.22 | Case-control | 5964 BCC; 1129 SCC; 1010 MM; 32 412 controls | No association | - | Association with amiloride | 1.79 (1.45−2.21) | No association | - |
| Ruiter et al.23 | Cohort | 10 692 individuals | No association | - | Not assessed | - | Association between loop diuretics in cumulatively high doses and BCC | 1.62 (1.09−2.42) |
| de Vries et al.8 | Case-control | 602 BCC; 409 SCC; 360 MM; 1550 controls | No association | - | Association | 1.66 (1.16−2.37) | No association | - |
| McDonald et al.24 | Cohort | 58 213 individuals, radiology technicians | Association only in overweight individuals | 1.22 (1.07−1.38) | Not assessed | - | Not assessed | - |
| Nardone et al.25 | Cohort | 27 123 individuals on ACEIs; 13 818 on ARBs; 15 166 on thiazide diuretics | Associated | 2.11 (1.60−2.79) | Association | 4.11 (2.66−6.35) | Association between ACEIs with BCC | 2.23 (1.78−2.81) |
| Association between ACEIs and SCC | 1.94 (1.37−2.76) | |||||||
| Association between ARBs and BCC | 2.86 (2.13−3.83) | |||||||
| Association between ARBs and SCC | 2.22 (1.37−3.61) | |||||||
| Su et al.26 | Cohort | 28 357 hypertensive individuals | Not assessed | - | Association with loop diuretics or MRAs | 1.32 (1.19−1.46) | No association | - |
| Robinson et al.27 | Case-control | 1567 BCC; 1599 SCC; 1906 controls | No association | - | Association | 1.3 (0.7−2.4) | No association | - |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; BCC: basal cell carcinoma; HCTZ: hydrochlorothiazide; MM: malignant melanoma; MRAs: mineralocorticoid receptor antagonists; NMSC: non-melanoma skin cancer; OR: odds ratio; SCC: squamous cell carcinoma.
The mounting volume of evidence led INFARMED, in coordination with the European Medicines Agency, to issue a communication for healthcare professionals, dated October 10, 2018, recommending that patients taking HCTZ should be informed of the risk of NMSC and advised to regularly check their skin. Patients should also be advised to limit their exposure to sunlight and UV rays and, in case of exposure, should use adequate protection in order to minimize the risk of skin cancer.
The use of HCTZ may also need to be reconsidered in patients who have experienced previous NMSC.12
This communication refers to two pharmacoepidemiologic studies conducted in Denmark and published in 2017 and 2018, which reveal a cumulative, dose-dependent association between HCTZ and NMSC.10,11 These studies were based on nationwide databases, including the Danish Cancer Registry and the Danish National Prescription Registry, and are noteworthy for their methodological rigor and sample size.
The study by Pedersen et al. included a population of 80 162 individuals with a new NMSC diagnosis (71 533 cases of BCC and 8629 cases of SCC), matched to a control group. Individuals on high doses of HCTZ, i.e. cumulatively higher than 50 000 mg, had an adjusted odds ratio (OR) of 1.29 (95% confidence interval [CI], 1.23−1.35) for BCC and 3.98 (95% CI, 3.68−4.31) for SCC. For very high cumulative HCTZ doses (>200 000 mg), even greater adjusted ORs were observed, estimated at 1.54 (95% CI, 1.38−1.71) for BCC and 7.38 for SCC (95% CI, 6.32−8.60).10
A study by Pottegård et al. published in 2017 assessed the relationship between HCTZ therapy and the risk of SCC of the lip. They matched 633 individuals with a histological diagnosis of lip cancer to a sample of 63,607 controls. A significant association was found among high users of the drug (>25 000 mg), with an OR of 3.9 (95% CI, 3.0−4.9). A clear increase in risk was also found with increasing cumulative dose, with an OR of 1.7 (95% CI, 1.5−1.9) for each additional 25 000 mg.11
Other studies in Denmark assessing the relationship between antihypertensive therapy and the risk of NMSC showed mixed results.19–21 In these studies, the association between thiazide diuretic use and increased risk of NMSC was less robust, and was only found when combined with mineralocorticoid receptor antagonists (MRAs). At the same time, other antihypertensive drugs were found to be associated with an increased risk of NMSC, notably furosemide, which was significantly associated with the risk of SCC in a study by Kaae et al.19
Apart from those carried out in Denmark, few studies in Europe have measured the association between HCTZ therapy and the risk of NMSC.8,22 In a cohort study conducted in the Netherlands, the only statistically significant relationship found was between high cumulative doses of loop diuretics and the risk of BCC.22 By contrast, another multicenter case-control study involving multiple European countries found an association between thiazide diuretic therapy and the risk of SCC.8
Studies performed in the US appear to show an association between thiazide diuretic therapy and the risk of NMSC, although their results are variable and sometimes contradictory.23–26 McDonald et al. and Nardone et al. reported an increased risk of BCC, but only in overweight individuals in the former.23,24 Nardone et al., Su et al. and Robinson et al. reported an association between these drugs and the risk of SCC.24–26 Similarly, studies in Europe also found associations with other antihypertensives. The study by Su et al. showed that renin-angiotensin-aldosterone system (RAAS) inhibitors, both ACEIs and ARBs, were associated with an increased risk of both histological types of NMSC.25
Two recently published meta-analyses aimed to identify a possible association between antihypertensive therapy and the risk of skin cancer.27,28 In a meta-analysis of 19 independent studies, Gandini et al. found no evidence of association between thiazide diuretic use and NMSC. However, an association was reported for calcium channel blockers (CCBs), which led to an increased risk of skin cancer (subtype unspecified) (OR 1.14, 95% CI, 1.07–1.21), and for beta-blockers (exclusively malignant melanoma [MM]) (OR 1.21, 95% CI, 1.05–1.40).27 In contrast to these findings, a meta-analysis by Tang et al. assessing 10 independent studies reported a statistically significant association between diuretic therapy and the risk of NMSC, with a greater OR for SCC (OR 1.40, 95% CI, 1.19–1.66) than for BCC (OR 1.10, 95% CI, 1.01–1.20). The risk of BCC was also shown to be associated with CCBs (OR 1.15, 95% CI, 1.09–1.21) and beta-blockers (OR 1.09, 95% CI, 1.04–1.15).28
Pottegård et al. recently conducted a study to determine whether an association existed between HCTZ therapy and the risk of MM, for which the OR was significant (OR 1.22, 95% CI, 1.09–1.36), with no increase for higher cumulative doses.29 Similarly, Nardone et al. found a statistically significant relationship between thiazide diuretic therapy and MM (OR 1.82, 95% CI, 1.01–3.82).24 Schmidt et al. and Jensen et al. had previously reported an increased risk of MM with ARBs (OR 1.44, 95% CI, 0.56–3.69) and indapamide (OR 3.30, 95% CI, 1.34–8.10), respectively.20,21
DiscussionOver the past decade, there has been growing interest in the potential role of antihypertensive therapy, particularly diuretics, in the risk of NMSC. Following the recent alert issued by INFARMED, we sought to collect the available evidence on the relationship between HCTZ and increased risk of these skin cancers.
When reviewing the existing literature, we found a series of studies suggesting an association between HCTZ therapy and the risk of SCC and, to a lesser extent, BCC. Recently published pharmacoepidemiologic studies conducted in Denmark have shown that this relationship appears to be cumulative and dose-dependent.10,11 Other studies conducted in Europe and in the US had suggested evidence of an association between HCTZ and the risk of NMSC, and this drug was found to be more strongly associated with SCC than BCC in all these studies. However, it should be noted that these results are somewhat variable, which may in part be explained by differences in methodology and study populations. During our research, we found references to a potential role in NMSC of antihypertensives belonging to other pharmacological classes, notably non-thiazide diuretics, CCBs, beta-blockers and RAAS inhibitors. It should nevertheless be noted that in many countries, including Denmark, HCTZ is marketed almost exclusively in combination with other antihypertensives, including MRAs.11 Monotherapy only effectively controls blood pressure in a small number of patients, so the individual role of each drug is often difficult to assess.14 Evidence of the impact of other antihypertensives on the risk of NMSC is mixed and unlikely to be generalizable. The large number of patients taking these drugs means additional studies are needed to clarify potential associations and their magnitude.
Although this was not the primary aim of this study, we encountered data suggesting a potential link between antihypertensive therapy and the risk of MM. Particularly interesting were the results of the study by Pottegård et al., which highlighted the need for further research to clarify the role of photosensitizing therapies such as HCTZ in the process of carcinogenesis.29
The methodological design of the studies examining a possible association between HCTZ and the risk of NMSC had certain limitations. In some cases, the influence of confounding factors which were not controlled for cannot be unequivocally ruled out.
The role of UV radiation in promoting photocarcinogenesis and the influence of skin phototype on the risk of skin cancer are well documented.8 In a significant number of studies, especially those conducted in Denmark, sun exposure habits, outdoor occupations and/or hobbies and history of sunburn were not assessed.10,11,19–21,24,26 In addition, skin phototype and other phenotypical features were assessed in only a few of the studies analyzed.8,22,23 The evidence of a relationship between HCTZ and the risk of NMSC comes primarily from studies conducted in countries with low UV radiation, such as Denmark, and no studies have been carried out in regions with a high UV radiation index.
A significant proportion of the studies mentioned in this article relied on databases to collect information on demographics, comorbidities and skin cancer diagnoses. The robust methodology of the Danish studies and the high quality of the evidence they obtained reflect the quality of their data recording systems, which are able to include reliable information on a large number of patients. Discrepancies between the results of the Danish studies and those from other countries, notably the US, may result from differences in the data collection process. However, other factors cannot be ruled out, particularly the influence of differing genetic and environmental factors, such as sun exposure habits, which were not examined in most studies.24
In the majority of studies, data on antihypertensive therapy were obtained from electronic drug prescription and dispensing records or from questionnaires. The use of electronic data is a potential source of bias, as actual treatment adherence cannot be assessed.
HCTZ appears to contribute to skin carcinogenesis through its photosensitizing action, and the latency period before it plays a part in the development and hence incidence of NMSC is likely to vary.17,22 Knowledge of the dosage and duration of treatment with HCTZ is essential to clarify its association with NMSC; however, this information was not available in some of the studies analyzed in this review, while in others any conclusions on the influence of HCTZ dose were limited by problems with data quality.8,21,24 It should also be noted that short follow-up times may have led to underestimation of the incidence of NMSC in some studies, given the long latency between the start of therapy with HCTZ and the development of skin cancer in some patients.
The risk of NMSC is increased in a range of conditions, including diabetes, human immunodeficiency virus infection, cancer and solid organ transplantation.30–32 It is impossible to rule out the possibility that these comorbidities influenced the results in some of the studies under analysis.8,19,22,23,25,26
HCTZ appears to have a more significant impact on the risk of SCC than of BCC; this suggests that its influence is associated with skin damage, which tends to be cumulative and chronic.10,11 Individuals with such damage are liable to develop precancerous dermatoses, particularly actinic keratosis, but this was not examined in most of the studies analyzed.
The closer monitoring that patients with hypertension are likely to receive, particularly in primary care, increases the likelihood that they will be diagnosed with NMSC.28 However, this type of bias appears less likely to have influenced our conclusions, considering the complexity of the associations between different antihypertensive drugs and the risk of skin cancer.
The high prevalence of HCTZ use has contributed to the reduction in mortality from cardiovascular disease observed in recent decades.33,34 Caution should be exercised when issuing alerts about this drug, bearing in mind the risk of causing alarm among healthcare professionals and, inevitably, patients, which could have a negative impact on treatment adherence and blood pressure management.
Alternative drugs should be considered for antihypertensive treatment in patients with a high risk of skin cancer, particularly those with a history of the disease.
ConclusionThe high incidence of NMSC and the large number of individuals on chronic HCTZ therapy may have major implications for public health. Although small, the increased risk reported for these cancers may result in a large number of patients being diagnosed, with significant clinical and economic repercussions.
The alert issued by INFARMED stresses the need to assess the benefit of HCTZ therapy on an individual basis, particularly when there is a history of skin cancer. It is particularly important to ensure the application of educational measures in these patients, such as avoiding sun exposure between 11 a.m. and 4 p.m., and encouraging preventive strategies such as wearing appropriate clothing and a hat and using sunscreen. Individuals on HCTZ therapy should be regularly monitored at follow-up visits for assessment of suspicious skin lesions and, if necessary, prompt referral to a dermatologist.
This article confirms a possible association between HCTZ and the risk of NMSC, but also reveals some limitations of studies in the literature. We believe that more prospective studies are needed with a robust methodological design that controls for potential risk factors, such as sun exposure and skin phototype, to identify the real extent of the relationship between antihypertensive therapy and skin cancer.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Garrido PM, Borges-Costa J. Terapêutica com hidroclorotiazida e risco de cancro cutâneo não melanoma: revisão da literatura. Rev Port Cardiol. 2020;39:160–166.