Both high-sensitivity CRP (hs-CRP) and uric acid (UA) levels are known to be increased in heart failure patients and are associated with poorer functional capacity and adverse outcome. The role of these markers in patients with mitral regurgitation (MR) is less clear. The aim of this study was to assess the relationship between hs-CRP, UA and organic MR. We also assessed whether hs-CRP and UA levels are correlated with symptoms of MR, severity of MR, LV remodeling and outcome during follow-up.
MethodsA total of 200 consecutive patients (87 men [43.5%]; mean age 61.6±12.5 years) with moderate or severe isolated and organic MR were included in the study. All the patients were assessed clinically and were managed and treated with standard medical therapy according to evidence-based practice guidelines. Patients were categorized according to New York Heart Association (NYHA) functional class. We assessed and graded the severity of MR using a multiparametric approach. hs-CRP was measured with chemiluminescent immunometric assay using an IMMULITE® 1000 autoanalyzer (Siemens, Germany). Serum UA levels were analyzed using a Cobas® 6000 autoanalyzer (Roche Diagnostics, Mannheim, Germany).
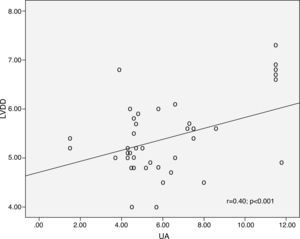
ResultsMean UA levels increased significantly with NYHA class: 4.46±1.58 mg/dl for patients in NYHA class I, 5.91±1.69 mg/dl for class II, 6.31±2.16 mg/dl for class III and 8.86±3.17 mg/dl for class IV (p<0.001). Mean UA levels also increased significantly with increased severity of MR (moderate 5.62±1.9 mg/dl, moderate to severe 5.56±1.2 mg/dl, severe 7.38±3.4 mg/dl, p<0.001). There was a significant correlation between UA level and left ventricular end-diastolic diameter (r=0.40; p<0.001), left ventricular end-systolic diameter (r=0.297; p=0.001) and left ventricular ejection fraction (LVEF) (r=0.195, p=0.036), whereas hs-CRP was not correlated with these parameters. In multivariate Cox proportional hazards analysis LVEF, NYHA class and UA levels were the only independent predictors of death.
ConclusionUA and hs-CRP levels can help identify patients with asymptomatic moderate or severe mitral regurgitation. UA levels may be useful to assess the extent of left ventricular remodeling and in the optimal timing of mitral valve surgery in certain subsets of patients.
Introdução É conhecido que tanto a proteína C-reativa de alta sensibilidade (PCR-as) como o ácido úrico (AU) estão elevados nos doentes com insuficiência cardíaca e, estão associados a pior capacidade funcional e a pior prognóstico. O papel destes marcadores nos doentes com regurgitação mitral (RM) é menos claro. O objetivo deste estudo foi o de avaliar a relação entre PCR-as, o AU e a RM orgânica. Também avaliamos se os níveis de PCR-as e de AU estão correlacionados com os sintomas da RM, com a severidade da RM, com a remodelagem do ventrículo esquerdo (VE) e com o prognóstico, durante o seguimento clínico.
MétodosUm total de 200 pacientes consecutivos (86 homens (43,5%); idade média 61,6±12,5 anos) com RM orgânica isolada, moderada ou severa, foram incluídos no estudo. Todos os doentes foram avaliados clinicamente e tratados com a terapêutica médica standard de acordo com a prática da medicina baseada na evidência e com as Guidelines. Os doentes foram classificados de acordo com a classe funcional da New York Heart Association (NYHA). Avaliamos e classificamos a gravidade da RM utilizando o modelo multiparamétrico. A PCR de alta sensibilidade foi quantificada com o teste imunométrico quemilumeniscente, usando um Autoanalizador IMMULITE® 1000 (Siemens, Germany). Os níveis séricos de AU foram determinados usando o Autoanalizador COBAS série 6000 (Roche® Diagnostics, Mannheim, Germany).
ResultadosOs níveis médios de AU aumentaram significativamente com a classe da NYHA: 4,46±1,58 mg/dl para Classe I da NYHA, 5,91±1,69 mg/dl para a classe II da NYHA, 6,31±2,16 mg/dl para Classe III da NYHA e 8,86±3,17 mg/dl para Classe IV da NYHA (p<0,001). Os valores médios do AU também aumentaram de forma significativa com o aumento da severidade da RM (moderada=5,62±1,9 mg/dl, moderada a severa=5,56±1,2 mg/dl, severa=7.38±3.4 mg/dl, p<0.001). Foi encontrada uma correlação significativa entre os níveis de AU e o DDVE (r=0.40; p<0.001, Figure 5), DSVE (r=0,297; p=0,001) e FEVE (r=0,195, p=0,036), enquanto a PCR-as não se correlacionou com esses parâmetros. Na análise multivariável de Cox, a FEVE, a classe da NYHA e os níveis de AU foram os únicos fatores preditivos independentes de morte.
ConclusãoOs níveis de AU e de PCR-as podem ajudar a distinguir os doentes com RM moderada a severa assintomática, dos sintomáticos. Os níveis de AU podem ser úteis na determinação da extensão da remodelagem ventricular e do tempo mais adequado para a realização da cirurgia da válvula mitral, em determinados grupos de doentes.
Primary mitral regurgitation (MR) covers all etiologies in which intrinsic lesions affect one or several components of the mitral valve apparatus.1 Surgery is recommended in patients with chronic severe MR if they have any symptoms or in asymptomatic patients with left ventricular (LV) dysfunction.1 When guideline indications for surgery are reached, early surgery is associated with better outcomes, since the development of even mild symptoms by the time of surgery is associated with deleterious changes in cardiac function after surgery.2,3 However, difficulties in detecting early LV dysfunction, accurately assessing the severity of valve involvement, or recognizing early cardiac symptoms often make it difficult to determine the optimal timing of mitral valve surgery.4 In many patients, the development of symptoms is clear, but in others, symptoms are difficult to assess because of inactivity. In some patients, it may also be unclear whether symptoms are related to severe MR or to comorbidities.5,6
Increased levels of high-sensitivity C-reactive protein (hs-CRP) are associated with poorer functional capacity and adverse outcome in patients with LV systolic dysfunction.7 Adelaide et al. reported that CRP elevation is also associated with the presence and severity of MR and with diastolic dysfunction in acute myocardial infarction. This suggests that inflammation is related to ventricular remodeling processes, independently of LV systolic function.8 As with hs-CRP, recent findings suggest that there is also a relationship between chronic heart failure (CHF) and uric acid (UA) levels.9,10 Elevated serum UA levels can predict poor survival in severe and mild–moderate CHF9,10 and may also predict exercise intolerance and inflammatory activation.11 Nozari et al. showed that elevated UA and hs-CRP are associated with heart failure (HF) among patients with myocardial infarction (MI) and their serum levels could be a predictor for the occurrence of severe HF in patients with MI.12 In addition, Yazicioglu et al. have demonstrated that elevated serum UA in patients with dilated HF is related to severity of functional MR.13
Although the association between hyperuricemia and hs-CRP with chronic heart failure has been recognized, the role of these biomarkers in MR patients is less clear. We therefore prospectively investigated the association between hs-CRP, UA and organic MR. We also assessed whether hs-CRP and UA levels are correlated with symptoms and severity of MR, LV remodeling and outcomes at one-year follow-up.
MethodsStudy populationA total of 200 consecutive patients with isolated and organic moderate or severe MR were included in the study. Exclusion criteria were as follows: a history of MI, previous cardiac surgery, presence of other valve disease (aortic valve disease, moderate or severe mitral stenosis, or significant right-sided organic valve disease), ischemic MR, significant liver or renal disease, chronic lung disease, malignant or hematologic disease, cardiomyopathies or pericardial diseases and concomitant inflammatory diseases such as infections and autoimmune disorders; poor echocardiographic acoustic window; and medication with hypouricemic agents. The study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Duzce University. All subjects provided written consent.
Clinical assessmentAll the patients were assessed clinically and were managed and treated with standard medical therapy according to evidence-based practice guidelines. Patients were categorized according to New York Heart Association (NYHA) functional class. Clinical assessment was performed by cardiologists who were blinded to UA and hs-CRP levels.
EchocardiographyIn all participants, transthoracic M-mode, two-dimensional, pulsed-wave, continuous-wave and color Doppler echocardiographic examinations were performed using a General Electric Vingmed Vivid 7 (GE Vingmed Ultrasound AS, Horten, Norway) using 2.5–3.5 MHz transducers. Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) diameter and left atrial (LA) diameter were determined from two-dimensional images, according to the American Society of Echocardiography guidelines.14 Left ventricular ejection fraction (EF) was calculated using the modified Simpson's method. Severity of MR was assessed from the regurgitant fraction and vena contracta width.15,16 In the case of equivocal findings between these two methods the MR score, as described by Thomas et al.,17 was calculated from visual assessment of MR jet penetration, mitral continuous-wave Doppler characteristics, LA size, pulmonary venous flow pattern, tricuspid regurgitation velocity, and proximal isovelocity surface area radius.5,14 Pulmonary artery systolic pressure (PAP) was calculated as the sum of the transtricuspid gradient and estimated right atrial pressure.18 The echocardiographers were blinded to patients’ symptom status and UA and hs-CRP levels.
Blood samplingVenous blood samples were drawn from the patients during a routine visit to the outpatient clinic. Whole blood count and routine biochemical tests were performed. hs-CRP was measured with chemiluminescent immunometric assay using an IMMULITE® 1000 autoanalyzer (Siemens, Germany). Serum UA levels were assessed using a Cobas® 6000 series autoanalyzer (Roche Diagnostics, Mannheim, Germany).
Follow-upClinical and echocardiographic assessments were performed at least yearly during a follow-up visit. Patients who died or underwent surgery were censored the same day, and those who remained alive were censored at the end of follow-up.
Statistical analysisThe statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean ± SD or median (25th–75th percentile), and categorical variables are presented as percentages and frequencies. The Student's t test and one-way ANOVA were used to compare normally distributed continuous variables in two or more groups. The Tukey test was used as a post-hoc test. The Kruskal-Wallis test was used to compare non-normally distributed continuous variables in more than two groups and the Mann-Whitney U test with Bonferroni adjustment was used for multiple comparisons. The chi-square test was used for categorical variables between two groups. Variables with a p value of <0.05 in univariate analysis were then entered into a multivariate Cox proportional hazards regression model to determine independent predictors of mortality during follow-up. Hazard ratios (HR) with 95% confidence intervals (CI) for risk factors are given. A p value of less than 0.05 was considered statistically significant.
ResultsOf the 200 patients with moderate, moderate to severe and severe MR included in the study, 54 were asymptomatic while 146 were symptomatic. Baseline demographic, clinical characteristics and laboratory parameters of patients with MR are listed and compared between asymptomatic and symptomatic patients in Table 1. Mean age was significantly higher in symptomatic than in asymptomatic MR patients (p<0.05). Male gender and hypertension were more prevalent in the symptomatic group than the asymptomatic group (p<0.05 for both). Treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blocker (ARBs) and diuretics was also more prevalent in symptomatic MR patients (all p<0.05).
Demographic, clinical characteristics and laboratory parameters of patients with mitral regurgitation and comparison between asymptomatic and symptomatic patients.
| All patients(n=200) | Asymptomatic(n=54) | Symptomatic(n=146) | p | |
| Mean age, years | 61.6±12.5 | 53.4±12.8 | 65.9±10.4 | <0.001** |
| Male, n (%) | 87 (43.5) | 14 (25.9) | 73 (50.0) | 0.002* |
| Hypertension, n (%) | 148 (74.0) | 31 (57.4) | 117 (80.1) | 0.002** |
| Smoking, n (%) | 28 (14.0) | 10 (18.5) | 18 (12.3) | 0.260 |
| Diabetes, n (%) | 39 (19.5) | 7 (13.0) | 32 (21.9) | 0.227 |
| BMI (kg/m2) | 29.4±5.4 | 29.4±3.4 | 29.7±6.4 | 0.710 |
| Atrial fibrillation, n (%) | 116 (58.9) | 28 (51.9) | 88 (61.5) | 0.257 |
| Echocardiographic findings | ||||
| LVEDD (mm) | 51.4±6.3 | 50.4±4.4 | 52.4±7.4 | 0.019* |
| LVESD (mm) | 36.2±7.3 | 34.4±6.8 | 37.5±8.2 | 0.013* |
| LA (mm) | 43.8±5.0 | 41.7±5.2 | 44.2±4.6 | 0.001** |
| Ejection fraction (%) | 54.1±11.5 | 60.2±9.4 | 51.0±12.2 | <0.001** |
| PAP (mmHg) | 36.5±9.5 | 33.5±6.8 | 37.3±10.2 | 0.013* |
| Mitral regurgitation severity | ||||
| Moderate | 106 (53.0) | 49 (90.7) | 57 (39.0) | <0.001** |
| Moderate to severe | 60 (30.0) | 5 (9.3) | 55 (37.7) | |
| Severe | 34 (17.0) | – | 34 (23.3) | |
| Laboratory findings | ||||
| Hemoglobin (g/dl) | 12.7±1.2 | 13.0±1.0 | 12.6±1.4 | 0.033* |
| BUN (mg/dl) | 18.8±7.7 | 17.8±6.8 | 19.9±9.3 | 0.123 |
| Serum creatinine (mg/dl) | 0.92±0.3 | 0.87±0.3 | 0.95±0.3 | 0.076 |
| Fasting glucose (mg/dl) | 113.2±42.4 | 98.7±12.2 | 104±18.5 | 0.023* |
| Total cholesterol (mg/dl) | 186.6±41.6 | 204±40 | 174±33 | <0.001* |
| HDL (mg/dl) | 47.7±12.9 | 48.6±11 | 45.3±13 | 0.119 |
| LDL (mg/dl) | 107.4±33.6 | 121±33 | 98±26 | <0.001** |
| Triglycerides (mg/dl) | 144.2±59.2 | 139±50 | 148±65 | 0.372 |
| hs-CRP (mg/l) | 5.26±6.38 | 2.56±1.9 | 5.9±6.8 | 0.004** |
| Uric acid (mg/dl) | 5.99±2.28 | 4.5±1.6 | 6.5±2.4 | <0.001** |
| Medication | ||||
| Beta-blockers, n (%) | 102 (51.8) | 23 (42.6) | 79 (55.2) | 0.150 |
| ACEIs/ARBs, n (%) | 152 (77.2) | 33 (63.1) | 119 (83.2) | 0.002** |
| Diuretics, n (%) | 97 (49.2) | 14 (25.9) | 83 (58.0) | <0.001** |
| CCBs, n (%) | 14 (7.1) | 4 (7.4) | 10 (7.0) | 0.920 |
| Digoxin, n (%) | 32 (16.2) | 6 (11.1) | 26 (18.2) | 0.283 |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; BMI: body mass index; CCBs: calcium channel blockers; hs-CRP: high-sensitivity C-reactive protein; LA: left atrial diameter; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; PAP: pulmonary artery pressure.
Values are mean ± SD (range) or n (%).
Patients in the symptomatic group had lower LVEF, higher PAP, increased LVEDD and LVESD and larger LA diameter compared to the asymptomatic group (all p<0.05). Moderate to severe and severe mitral regurgitation was significantly more prevalent in the symptomatic group (p<0.001). Hemoglobin, total cholesterol, and low-density lipoprotein (LDL) cholesterol levels were significantly lower, while fasting glucose was higher, in the symptomatic group (all p<0.05).
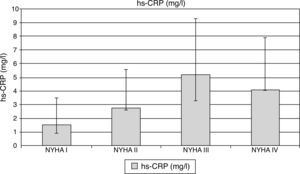
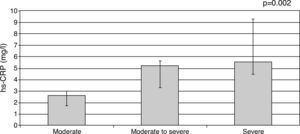
hs-CRP was significantly elevated among symptomatic MR patients compared to asymptomatic patients (5.9±6.8 vs. 2.56±1.9 mg/l; p=0.004). Median hs-CRP levels were increased significantly with increase in NYHA class (NYHA class I: 1.50 mg/l [0.90–3.50], class II: 2.73 mg/l [2.59–6.57], class III: 5.20 mg/l [3.30–9.30], and class IV: 4.07 mg/l [4.03–7.92], p<0.001; Figure 1). A similar correlation was also found with MR severity, median hs-CRP levels being significantly increased with more severe MR (moderate: 2.62 mg/l [1.70–2.95], moderate to severe: 5.20 mg/l [3.30–5.6], and severe: 5.53 mg/l [4.50–9.30], p=0.002; Figure 2).
High-sensitivity C-reactive protein levels according to NYHA functional class. Top of the bar represents median and whiskers represent the 25th and 75th percentile of concentrations. hs-CRP: high-sensitivity C-reactive protein.
P1: NYHA I vs. NYHA II; P2: NYHA I vs. NYHA III; P3: NYHA I vs. NYHA IV; P4: NYHA II vs. NYHA III; P5: NYHA II vs. NYHA IV; P6: NYHA III vs. NYHA IV.
p1<0.001, p2=0.001, p3<0.001, p4=0.008, p5=0.062, p6=0.794.
High-sensitivity C-reactive protein levels according to degree of mitral regurgitation. Top of the bar represents median and whiskers represent the 25th and 75th percentile of concentrations. hs-CRP: high-sensitivity C-reactive protein.
P1: moderate vs. moderate to severe; P2: moderate vs. severe; P3: moderate to severe vs. severe.
p1=0.99, p2=0.004, p3=0.004.
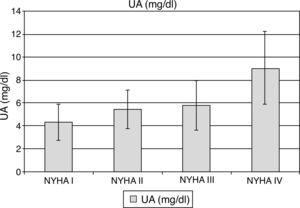
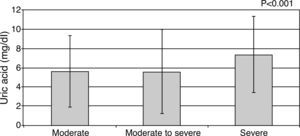
UA levels were significantly elevated among symptomatic compared to asymptomatic patients (6.5±2.4 vs. 4.5±1.6 mg/dl; p<0.001). Mean UA levels increased significantly with NYHA class: 4.46±1.58 mg/dl for NYHA class I, 5.91±1.69 mg/dl for class II, 6.31±2.16 mg/dl for class III and 8.86±3.17 mg/dl for patients in class IV (p<0.001; Figure 3). Mean UA levels also increased significantly with increased severity of MR (moderate: 5.62±1.9 mg/dl, moderate to severe: 5.56±1.2 mg/dl, severe: 7.38±3.4 mg/dl, p<0.001; Figure 4). There was a significant correlation between UA level and LVEDD (r=0.40; p<0.001, Figure 5), LVESD (r=0.297; p=0.001) and LVEF (r=0.195, p=0.036), whereas hs-CRP was not correlated with these parameters.
Uric acid levels according to NYHA functional class. Top of the bar represents mean and whiskers represent standard deviations. UA: uric acid.
P1: NYHA I vs. NYHA II; P2: NYHA I vs. NYHA III; P3: NYHA I vs. NYHA IV; P4: NYHA II vs. NYHA III; P5: NYHA II vs. NYHA IV; P6: NYHA III vs. NYHA IV.
p1=0.029, p2=0.010, p3<0.001, p4=0.840, p5<0.001, p6=0.001.
Uric acid levels according to degree of mitral regurgitation. Top of the bar represents median and whiskers represent the 25th and 75th percentile of concentrations. UA: uric acid.
P1: moderate vs. moderate to severe; P2: moderate vs. severe; P3: moderate to severe vs. severe.
p1<0.001, p2<0.001, p3=0.123
In addition, hsCRP levels were nearly significantly higher (5.5±2.5 vs. 4.2±3.6 mg/l, p=0.053), and UA levels were significantly higher (7.6±2.8 vs. 5.5±1.9 mg/dl, p<0.001) in patients who needed surgery than in those who did not according to current ESC guidelines on valvular heart disease.1
Mean follow-up was 437.1±133.9 (20–545) days. During follow-up 21 (10.5%) patients died and 26 (13%) underwent surgery. Increased UA levels were significantly associated with an increased risk of death during follow-up, along with male gender, increased serum creatinine, reduced LVEF, more severe MR and higher NYHA functional class (Table 2). In multivariate Cox proportional hazards analysis LVEF, NYHA class and UA levels were the only independent predictors of death (Table 2).
Results of univariate and multivariate Cox proportional hazard analysis of mortality in patients with mitral regurgitation.
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender | 0.172 | 0.058–0.511 | 0.002** | 3.521 | 0.764–16.216 | 0.216 |
| NYHA class | 10.296 | 5.136–20.640 | <0.001** | 3.803 | 1.243–11.637 | 0.019* |
| Uric acid | 1.043 | 1.022–1.065 | <0.001** | 1.050 | 1.015–1.086 | 0.005** |
| Serum creatinine | 7.865 | 2.792–22.154 | <0.001** | 2.715 | 0.153–17.89 | 0.618 |
| Mitral regurgitation | 6.469 | 3.207–13.049 | <0.001** | 1.436 | 0.391–5.282 | 0.586 |
| Ejection fraction | 0.882 | 0.839–0.927 | <0.001** | 0.936 | 0.878–0.998 | 0.045* |
NYHA: New York Heart Association.
We found a significant increase in UA and hs-CRP levels in patients with higher degrees of MR and NYHA class. We also found that increased UA level, reduced LVEF and higher NYHA class were significantly associated with an increased risk of death during follow-up.
Surgery is indicated in patients who have symptoms due to chronic MR but no contraindication to surgery. The management of asymptomatic patients is controversial as there are no randomized trials to support any particular course of action.2,19 In some patients, including those who are elderly, physically inactive or obese, it is difficult to assess symptoms because of inactivity. On the other hand, in some patients, such as those with pulmonary disease, it may be unclear whether symptoms are related to severe MR or comorbidities.5,6 Noninvasive markers that show early changes in the cardiovascular system would therefore be helpful in assessing patients with MR.6 Several studies have reported the value of elevated BNP levels to identify asymptomatic MR patients at higher risk of developing heart failure, LV dysfunction or death in mid-term follow-up.5,20,21 A recent study showed a significant correlation between proadrenomedullin levels and degree of MR and NYHA functional class.22 It has been reported that patients with ischemic heart disease, LV systolic dysfunction and dilated cardiomyopathy with elevated hs-CRP have a worse prognosis.23,24 Two previous studies indicated that CRP predicts heart failure and death after MI.25,26 In our study, we found that hs-CRP levels were significantly higher among symptomatic than in asymptomatic patients. Additionally, hs-CRP levels were found to be significantly increased with higher degrees of MR and NYHA class. Hence, hs-CRP may be of value for identification of advanced stages of MR when other tools are not available or less informative. We also believe that hs-CRP may have a potential role for monitoring patient response to medication or surgery in follow-up. In agreement with our study, Arruda-Olson et al. reported that the presence of diastolic dysfunction and moderate or severe MR was associated with higher CRP levels. After further adjustment for infection within two weeks prior to MI, the association between CRP and diastolic dysfunction was no longer significant, but the association between CRP and MR remained.8 In contrast to studies in HF patients, hs-CRP was not correlated with LV dimensions or LVEF.
Pathways of UA production and metabolism may have a considerable impact on CHF pathophysiology and its clinical picture.27,28 It has been shown that patients with CHF are hyperuricemic compared to controls, independently of the effects of diuretics, renal impairment and other metabolic factors.9 UA levels were significantly correlated with LV systolic and diastolic dysfunction in patients with CHF.29,30 Hyperuricemia is a constant finding in CHF, its prevalence increasing with disease severity.31,32 Elevated serum UA may itself convey prognostic information in CHF.33 Anker et al. demonstrated an independent and graded relationship between serum UA levels and survival in patients with moderate to severe CHF.10 It has been shown that in patients with mild to moderate CHF, elevated serum UA levels are strongly related to death, and this relationship is independent of CHF severity and impaired renal function.33 In the same study, hyperuricemia was reported to predict exercise intolerance and was a marker of inflammatory activation in CHF. Serum UA levels increased significantly in parallel with CHF severity expressed as NYHA class.33 In our study, UA was significantly higher among symptomatic MR patients than in asymptomatic patients. This may help to identify asymptomatic patients in follow-up. We also found that mean UA levels increased significantly with NYHA class and higher degrees of MR. These findings suggest that UA is a potential biomarker for the assessment of therapeutic response to either medication or surgical intervention in follow-up. In agreement with previous studies in patients with heart failure, UA was significantly correlated with LVEDD, LVESD and LVEF. Therefore, we believe UA levels may be useful to assess the extent of LV remodeling and in the optimal timing of mitral valve surgery in certain subsets of patients.
As a limitation of the study, we cannot rule out the impact of diuretic dose on UA.
ConclusionUA and hs-CRP levels can help to distinguish patients with asymptomatic moderate to severe MR from symptomatic patients. UA levels may be useful to assess the extent of LV remodeling and the optimal timing of mitral valve surgery in certain subsets of patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.