Postoperative pulmonary complications are a common cause of morbidity and mortality in patients undergoing cardiac surgery, leading to an increase in length of hospital stay and healthcare costs.
This systematic literature review aims to determine whether patients undergoing cardiac surgery who undergo preoperative breathing exercise training have better postoperative outcomes such as respiratory parameters, postoperative pulmonary complications, and length of hospital stay.
Systematic searches were performed in the CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Clinical Answers, Cochrane Database of Systematic Reviews, MEDLINE and MedicLatina databases. Studies were included if they examined adult patients scheduled for elective cardiac surgery, who underwent a preoperative breathing exercise training aimed at improving breathing parameters, preventing postoperative pulmonary complications, and reducing hospital length of stay. This systematic review was based on Cochrane and Prisma statement recommendations in the design, literature search, analysis, and reporting of the review.
The search yielded 608 records. Eleven studies met the inclusion criteria. Ten studies were randomized controlled trials and one was an observational cohort study. Data from 1240 participants was retrieved from these studies and meta-analysis was performed whenever possible.
A preoperative breathing intervention on patients undergoing cardiac surgery may help improve respiratory performance after surgery, reduce postoperative pulmonary complications and hospital length of stay. However, more trials are needed to support and strengthen the evidence.
As complicações pulmonares pós-operatórias são uma causa comum de morbilidade e mortalidade em pessoas submetidas a cirurgia cardíaca, conduzem ao aumento do tempo de internamento hospitalar e custos associados.
Esta revisão sistemática da literatura tem como objetivo determinar se as pessoas a aguardar cirurgia cardíaca que participam numa intervenção de exercícios respiratórios apresentam melhores resultados pós-operatórios em relação aos parâmetros respiratórios, às complicações pulmonares pós-operatórias e ao tempo de internamento.
Pesquisas sistemáticas foram realizadas nas bases de dados Cinahl, Cochrane Central Register of Trials Controled, Cochrane Clinical Answers, Cochrane Database of Systematic Reviews, Medline e MedicLatina. Os estudos eram incluídos quando compreendiam pessoas adultas inscritas para cirurgia cardíaca eletiva, submetidas a uma intervenção baseada em exercícios respiratórios, pré-operatória, com o objetivo de melhorar os parâmetros respiratórios, prevenir complicações pulmonares pós-operatórias e reduzir o tempo de internamento. Esta revisão foi fundamentada nas recomendações das declarações Cochrane e Prisma, no desenho, na pesquisa da literatura, análise e no relatório da revisão.
Da pesquisa resultaram 608 artigos. Onze estudos cumpriram os critérios de inclusão, dos quais dez são ensaios clínicos aleatorizados e um é um estudo de coorte observacional. Os dados de 1240 participantes foram analisados e a metanálise foi realizada sempre que possível.
Uma intervenção baseada em exercícios respiratórios aplicada a pessoas a aguardar cirurgia cardíaca pode ajudar a melhorar o desempenho respiratório após cirurgia, reduzir complicações pulmonares pós-operatórias e tempo de internamento. No entanto, são necessários mais estudos para fortalecer a evidência encontrada.
Cardiovascular diseases are the main cause of mortality and hospital admission.1 Cardiac surgery emerges as a form of treatment when conservative ways are no longer a viable resource. The most frequent cardiac surgeries are heart valve replacements and coronary artery bypass grafting (CABG). Although cardiac surgery uses advanced techniques and materials that allow the procedure to be safe, there are still risks associated with it; the reported incidence of postoperative complications varies from 5% to 90%, depending on how the complications are defined.1–3 Postoperative pulmonary complications (PPC) occur frequently after cardiac surgery, caused by surgical procedures, anesthesia, and pain that impairs chest mobility and lung expansion.4 PPC may affect up to 70% of cases, specifically atelectasis and pneumonia at 24.7%, and hypoxemia and pleural effusion at47.5%, and it is associated with limited ability to take deep breaths, lung atelectasis, and pulmonary disfunction.5,6 Such complications increase morbidity, mortality and health care costs.4
Cardiac surgery is usually performed via a median sternotomy; sternal pain is common in patients, and reported to be a risk factor in the first postoperative days, causing the patient to adopt a restrictive and shallow breathing pattern.6,7
If a patient undergoes cardiac surgery with preoperative respiratory dysfunction, it is more likely for him/her to maintain postoperative mechanical ventilation for longer after heart valve surgery; decreased respiratory muscle strength has been described as an important factor leading to impaired functional capacity after CABG.8,9
Respiratory muscle strength two months after cardiac surgery is not impaired when compared to preoperative values.10 Despite this, Riedi et al. reported an 11% reduction in maximal inspiratory pressure five days after surgery,11 and Morsch reported a 36% reduction six days after surgery.12 These results on the early postoperative period may be due to sternal pain and lack of ability to perform the exercises correctly.10 A sternotomy reduces chest wall compliance and the ability to breathe properly.10
Breathing therapy exercises are a well-accepted intervention, introduced as treatment for cardiac surgery patients. The aim of these exercises, in the early postoperative period, is to reduce the risk of PPC, functional capacity impairment, and length of hospital stay (LHS) due to altered pulmonary function.
Preoperative exercises have been known to be effective in reducing postoperative complications; there are systematic reviews on both preoperative methods and breathing therapy combined with physical exercises, which confirm the positive effect on functional capacity, decreased PPC and LHS after cardiac surgery.13 In another systematic review, the literature consulted did not support the hypothesis that preoperative physical activity alone is associated with better cardiac surgical outcomes.14 However, breathing therapy through inspiratory muscle training (IMT) alone appears to be efficient in decreasing PPC after cardiac surgery.15
Therefore, the purpose of this systematic review was to discover whether breathing therapy (any breathing exercise) performed preoperatively in persons awaiting cardiac surgery is effective, when comparing the following postoperative outcomes: respiratory parameters, PPC and LHS, among cardiac surgery patients included in a preoperative breathing therapy program and those who were not ncluded in any program preoperatively.
MethodsThis systematic review was based on the Cochrane and Prisma statement recommendations for the design, literature search, analysis, and reporting of the review.16,17
Search strategyAt first, studies were searched in the CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Clinical Answers, Cochrane Database of Systematic Reviews, MEDLINE and MedicLatina databases, and articles were searched from inception to October 2019. The search strategy combined terms related to the population (cardiac surgery, heart surgery) with terms for the intervention (preoperative, breathing exercises, breathing therapy, inspiratory muscle training) and expected outcomes (length of stay, postoperative pulmonary complications).
Studies were included if they examined patients registered for elective cardiac surgery, who underwent a preoperative breathing exercises intervention aimed at improving breathing parameters, preventing PPC and reducing LHS.
Studies were screened by headings, and then abstracts provided more precise information about the population or intervention used.
Reference lists and citations of included articles and any relevant systematic review were reviewed to identify publications not retrieved by the database search.
Eligible studies were reviewed, and data was extracted to assess the risk of bias using The Cochrane Risk of Bias Tool.17
Inclusion and exclusion criteriaStudies were included if they compared adult (≥18) cardiac surgery patients undergoing CABG and/or valve repair/replacement. Studies with patients undergoing heart transplantation or other types of cardiac surgical procedures were excluded. All studies were able to compare patients who had preoperative breathing therapy with patients who haven’t been included in any preoperative therapy intervention, who were submitted to a placebo treatment or only received instructions and education on the day before surgery, including assessments about surgery outcomes, PPC or LHS.
Studies were excluded if preoperative breathing therapy was combined with any physical activity training intervention.
Quality assessmentTwo authors independently reviewed all potential studies for inclusion against the eligibility criteria. To ensure the quality of the studies, only randomized clinical trials (RCT) and cohort studies were included according to levels of methodological quality18 (Table 1).
Levels of methodological quality.
| Level | Explanation |
|---|---|
| A1 | Systematic review of at least two independently conducted studies of A2 level |
| A2 | Randomized double-blind comparative clinical studies of good quality and sufficient size |
| B | Comparative studies but not with all features listed under A2 |
| C | Non-comparative studies |
| D | Expert opinion |
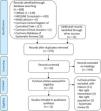
The database searches and the additional snowball search resulted in 608 citations (Figure 1). Through additional screening from other reviews and from relevant articles that were found, another 12 citations emerged. After removing the duplicates, 576 articles remained. These articles were screened, after which 544 were excluded based on their headings: other therapy interventions not related to cardiac surgery (456), other cardiac procedures (8), subjects younger than 18 (13), systematic literature reviews (23), not right intervention (44).
The abstracts of the remaining 35 articles were scrutinized; those that assessed the effectiveness of breathing therapy only in the postoperative period, and those that had any kind of preoperative intervention other than breathing therapy, if the patient was not assessed after having undergone surgery and systematic reviews, were also excluded. Eleven articles were retrieved, ten in full text and one in a published poster; these were also assessed for potential eligibility. All eleven studies were included in the qualitative analyses.
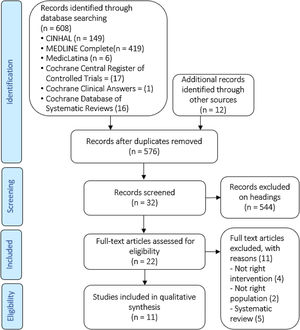
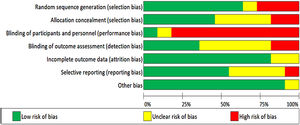
Risk of bias in included studiesThe included studies were assessed for bias. The Cochrane Risk of Bias Tool was used in all included studies (Figures 2 and 3).17
Seven studies used adequate methods for random sequence generalization,19–25 three other studies did not use random sequence generalization26–28 and one of them failed to explain how this process was handled.29 Five trials used allocation concealment,19–21,23,24 two failed to do this25,27 and four did not report it.22,26,28,29
Due to the nature of the intervention, it was difficult to blind the participants and personnel in nine trials. Despite this, one group reported using a sham intervention. The control group used the same inspiratory muscle training device as the intervention group, but no resistance was applied to it.28 Carvalho, Bonorigo & Panigas failed to report this.29
Four studies blinded the assessment of the outcomes,19–21,24 this was achieved by having a different researcher collect medical records. The rest of the studies reported no blinding,23,27or it was unknown whether this was taken into account.22,25,26,28,29
Nine of the trials reported information regarding outcome data,19–25,27and the other two studies did not provide information on this.28,29
Six of the studies showed all data, including non-significant19,20,22–25 data, only Valkenet et al. failed to do this,27 and in four other studies this was dubious.21,26,28,29
None of the studies presented any other risk of bias, except for Carvalho, Bonorigo & Panigas whose report was unclear.29
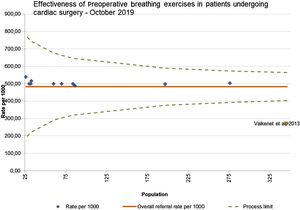
A funnel plot of all 11 studies, was used to check publication bias; minimal asymmetry indicates lack of publication bias (Figure 4).17 The study conducted by Valkenet et al.27 falls outside the limits of confidence, most likely due to lower methodological quality,17 as it is the only observational cohort study as the all the other ten are RCTs.
Study characteristicsThe articles were then submitted for critical appraisal; ten studies were randomized controlled trials and one of them was a prospective cohort study for preventive breathing therapy interventions in subjects undergoing cardiac surgery; 1240 patients were included in the analysis. Table 2 describes all the included studies. The median sample size of the 11 selected studies was 70 [range: 26-346], which could be divided into intervention group (IG) 35 [range: 14-139] and control group (CG) 35 [range: 12-252]. Median age of all patients included was 62 years old [range: 54-71]. Male gender accounted for the main subjects of each trial with a median percentage of 69% [range: 50%-100%], whereas female gender presented a median percentage study size of 31% [range: 0%-50%].
Summary data from 11 studies.
| Author, year | Study design | Surgery | ParticipantsN (Age±SD) (men/woman) | Intervention | Comparison | Outcomes | Study quality |
|---|---|---|---|---|---|---|---|
| (Carvalho et al., 2011) | RCT | CABG | N=32IG 16(62±9.9)(62.5%/37.5%)CG: 16(62±10.9)(68.8%/31.3%) | IMT in IG was performed with the set Threshold IMT with workload set to 30% of the MIP, during the 2 weeks prior to surgery. Training was performed seven days/week, twice day, three sets of 10 repetitions. | Unknown. | Pneumonia:IG: 5.3% vs CG: 12.3%, p=0.04Atelectasis:IG: 18.7% vs. CG: 43.2%, p=0.02Pleural effusion:IG: 12.5% vs. CG: 31.3%IMT was efficient increasing respiratory muscle strength (MIP/MEP) and function capacity (6MWT), reducing PPC. | B |
| (Chen et al., 20019) | RCT | CABG and/or valve | N=197IG: 98 (61.68±7.73)(74.5%/25.5%)CG: 99 (61.68±8.12)(68.7%/31.3%) | Threshold IMT device was used for IMT – the IG received IMT at 30% of MIP for 20 minutes twice a day the last five days with supervision by a physical therapist. Resistance was increased steadily, based on the rate of perceived exertion on the Borg scale. If the rate was less than 5, the resistance of the inspiratory threshold trainer was increased by 5% at a time. Patients were instructed to maintain diaphragmatic breathing with this device for 5 breaths and maintain this pattern for 20 minutes, twice a day. | Both groups performed abdominal breathing training, twice per day at 20 minutes each, lin five days before surgery.CG used the same protocol as the participants in the IG for the same number of repetitions, frequency, duration and supervision, but the intensity was fixed at the minimum load of the device (9 cmH2O). | MIP:IG: 100.8±23.36 vs. CG 93.12±23.12, p<0.001LHS:IG: 7.51±2.83 vs. CG: 9.38±3.10, p=0.039PPC grade≥2:IG: 10.2 vs. CG: 27.3, p=0.002Pneumonia:IG: 3.1 vs. CG: 7.1, p=0.321 | A2 |
| (Ferreira et al., 2009) | RCT | CABG or valve surgery | N=30IG: 15 (62.47±8.06)(60%/40%)CG: 15 (63.91±7.93)(86.7%/13.3%) | General advice about surgery and postoperative care, advised to stop smoking and not to smoke before surgery. Deep inspiration exercises and daily walks within their own limits.Patients had to perform five series of 10 calm and deep inspirations with at least one-minute intervals between the series, with the incentive of a respiratory instrument “Threshold IMT” (Respironics, Cedar Grove, NJ, USA), with a load of 40% of MIP (D0)15. The series were to be repeated thrice daily, while waiting for the surgery. | CG received general advice for pre-surgery. Did not perform IMT exercises with Threshold. | Pneumonia:IG: 1 (6.7%) vs. CG: 0 (0%), NSThe IMT program resulted in improved forced vital capacity and maximal voluntary ventilation, although its clinical benefits were not demonstrated. | B |
| (Hulzebos et al., 2006) | RCT-pilot | CABG | N=26IG: 14 (70.14±9.9)(50%/50%)CG: 12 (70.5±10.1)(50%/50%) | Subjects in the IG trained daily at home, seven times/week, for at least two weeks before surgery. Each training session consisted of 20 minutes of IMT. One session a week was supervised by the same physical therapist. | Education about early mobilization and coughing with wound support one day before surgery (usual care). | Pneumonia:IG: 1 (7.1%) vs. CG: 1 (8.3%), NSAtelectasis:IG: 2 (14.2%) vs. CG: 6 (50%), p=0.05LHS:IG: 7.93±1.94 vs. CG: 9.92±5.78, p=0.24IMT significantly improves inspiratory muscle strength (increase of 36%) in the preoperative period and seems to prevent postoperative atelectasis. | B |
| (Hulzebus et al., 2006) | RCT | CABG (at high risk of PPC) | N=276IG: 139 (66.5±9.0)(77.7%/22.3%)CG: 137 (67.3±9.2)(78.1%/21.9) | IG received preoperatively individualized exercises, IMT, incentive spirometry; education in active cycle of breathing techniques; and forced expiration techniques. The intervention group trained daily, seven times a week, for at least two weeks before the actual date of surgery. Each session consisted of 20 minutes of IMT, which was performed six times/week without supervision and once a week with supervision, when the strength and endurance of the inspiratory muscles after each week of training was measured. | Instruction in deep breathing, coughing and early mobilization one day prior to surgery (usual care). | PPC grade ≥2:IG: 25 (18%) vs. CG: 48 (35%), p=0.02Pneumonia:IG: 9 (6.5%) vs. CG: 22 (16.1%), p=0.01LHS:IG: 7 (range 5-41) vs. CG: 8 (range 6-70), p=0.02Physical therapy with IMT administered to patients at high risk of PPC before CABG surgery was associated with an increase in inspiratory force and a decrease in the incidence of PPC and LHS. | A2 |
| (Leguisamo et al., 2005) | RCT | CABG | N=86IG: 42 (59.3)(73.8%/26.2%)CG: 44 (60.6)(80.95%/19.05) | IG was assessed and coached for at least two-weeks before surgery, written guidelines on ventilatory exercises and coughing were given to continue the exercises at least twice a day until hospital admission. An individual weekly meeting was scheduled to monitor and provide guidance on breathing exercises: 1) diaphragmatic ventilatory pattern; 2) ventilatory pattern with inspiration split in two parts; 3) ventilatory pattern with inspiration split in three parts, performed in two series of 10 repetitions of each type of exercise. | CG received guidance and was evaluated 24h before surgery. | No statistically significant difference in PPC between groups. LHS:IG: 11.77±6.26 vs. CG: 14.65±6.61, p<0.005 | B |
| (Shakuri et al., 2014 | RCT | CABG | N=60IG: 30 (54.4±10.8)(63.3%/36.7%)CG: 30 (59.3±10.45)(90%/10%) | Two-week period before the surgical operation, 15 sessions, consisting of exercises and auxiliary activities for extension and rotation of thoracic vertebrae, breathing exercises, exercises to expand lung lobes, instruction of incentive spirometer equipment, extension exercise for thoracic cavity muscles and muscles with a role in breathing (aerobic exercises) for 25 minutes at a constant low speed for all the patients. | CG received rehabilitation care only after surgery (usual care). | FEV1:IG: 80.0±12.4 vs. CG: 73.8±13.16MWT, meter/spO2%:IG 97.7±16.39/96.4±5.34 vs. CG 76.3±20.5/97.1±1.4Spirometry differences were significant and higher in IG.Respiratory performance based on 6MWT parameters showed greater difference in the means of spO2 and distance walked in IG. | B |
| (Sobrinho et al., 2014) | RCT | CABG | N=70IG: 35 (58.9±9.53)(65.7%/34.3%)CG: 35 (61.4±8.43)(82.9%/17.1%) | IG performed under supervision, once a day, during the time that preceded the surgery, breathing exercises (breathing in time, deep breathing followed by prolonged expiration, sustained maximal inspiration with apnea of six seconds, and diaphragmatic breathing associated with the mobilization of the upper limbs) and breathing exercises with threshold - IMT® at an intensity of 40% of the initial maximal inspiratory pressure with three sets of ten repetitions, respecting two-minute intervals between each series. | Received guidelines on ward (usual care). | MIP PO5:IG: 100 vs. CG: 80, p<0.05LHS:IG: 8460 min (10 080-6730) vs. CG: 9970 (19 580-6730), p<0.001Decrease in LHS of approximately 25 hours in IG. | B |
| (Turky et al., 2017) | RCT | CABG | N=33IG: 17 (56.9±3.75)(100% males)CG: 16 (56.95±4.35)(100% males) | IG received preoperative IMT via a threshold load inspiratory muscle trainer (30% of their MIP, the resistance increased based on the RPE dyspnea score reported, if the RPE was less than resistance of the inspiratory threshold training increased incrementally by 2 cmH2O. The resistance was not changed if the perceived exertion was rated from 6 to 8, the resistance was decreased by 1 to 2 cmH2O if the perceived exertion was rated from nine to 10. The patients were encouraged to complete three sets of 10 breaths as slow maximal inspirations, with 30-60 second pause between each set, twice daily.Education on efficient coughing and early mobilization to use postoperatively. | Preoperative education (usual care) without training by the IMT. | MIP:PO2, NSPO8: IG: 71.58 vs. CG 37.44, p=0.001SpO2%:PO2 - IG: 97.1 vs. CG 95.8, p=0.001PO8 - IG: 98.85 vs. CG 97.85, p=0.001LHS:9.05±0.75 days in both groups, NSPreoperative IMT improved the alveolar-arterial gradient of patients who underwent CABG operation, which reduced the risk of PPC. | B |
| (Valkenet et al., 2013) | Observational Cohort Study | CABG and valve surgery (at high risk of PPC) | N=346IG: 94 (66.8±12.5)(61.7%/38.3%)CG: 252 (68.4±9.3)(68.3%/31.7%) | Patients visited the outpatient clinic at least 2 weeks before surgery. Received instructions and education concerning postoperative deep breathing exercises, incentive spirometry, coughing with wound support, and the importance of early postoperative mobilization.IG received one instruction session and was instructed to perform IMT at home until surgery. | Received instructions and education concerning postoperative deep breathing exercises, incentive spirometry, coughing with wound support, and the importance of early postoperative mobilization. They did not perform IMT, as there was not enough time before the surgery. | Pneumonia:IG: 1.1% vs. CG: 3.2%Ventilation time:IG: 7 [5-9] vs. CG 7 [5-10] hoursLOS (ICU):IG: 23 [21-24] vs. CG: 23[21-25] hoursLHS:IG: 7[6-11] vs. CG: 7 [5-9] daysIt cannot be stated that IMT in routine care resulted in less postoperative pneumonia, decreased ventilation time or decreased length of stay. | B |
| (Weiner et al., 1998) | RCT | CABG | N=84(69%/31%)IG: 42(59.2±3.8)CG: 42(63.8±3.1) | IMT resistance (Threshold inspiratory muscle trainer), starting at 15% of patient MIP up to 60% (increased incrementally 5% per session) of MIP, six days/week, for two to four weeks before the operation, 30 min training (depending on the date of surgery).Each session consisted of 0.5 h under supervision. | Sham training. IMT with no resistance, six days/week, two to four weeks. | Pneumonia:IG: 1 (3.4%) vs. CG: 3 (7.14%), NSPleural effusion:IG 5 (11.9%) vs. CG 3 (7.1%)Hemidiaphragmatic paralysis:IG: 2 (4.8%) vs. CG: 3 (7.1%)IMT for a period of 2 to 4 weeks before surgery resulted in a significant increase in inspiratory muscle strength and endurance before the surgery and led to significantly better blood gases and pulmonary function after the operation. | A2 |
CG: control group; CABG: coronary artery bypass graft; FEV1: forced expiratory volume in 1 second; ICU: intensive care unit; IG: intervention group; IMT: inspiratory muscle training; LOS: length of stay; LHS: length of hospital stay; MEP: maximal expiratory pressure; MIP: maximal inspiratory pressure; NS: not significant; O: postoperative day; PPC: postoperative pulmonary complications; RCT: randomized controlled trial; RPE: rate of perceptive exertion; SD: standard deviation; spO2: blood oxygen saturation; VM: minute volume; 6MWT: six minute walk test.
Eight studies only had CABG patients as their population20–25,28,29 and three studies investigated patients undergoing CABG and/or valve surgery.19,26,27
The studies included only breathing related interventions; either diaphragmatic breathing, inspiratory muscle training, or resorting to incentive spirometer equipment. The breathing interventions could be combined or single use. All studies aimed to improve the quality of respiratory performance after cardiac surgery, five studied respiratory parameters,19,23–25,28 11 searched for the prevalence of PPC,19–29 and another six studies also measured LHS.19–22,25,27 Furthermore, three studies used a single intervention (breathing therapy),20,22,23 four other studies used IMT (threshold),19,25,28,29 one used incentive spirometer associated with breathing exercises,26 another IMT (threshold) and breathing exercises,24 and two others a combination of multiple breathing therapy exercises.21,26
In high quality studies (quality level A2), there was an improvement in inspiratory muscle strength that led to better blood gases and pulmonary function28; there was also a reduction among high risk patients of the incidence of PPC and LHS.19,21 In fair quality studies (quality level B), the interventions used, achieved an improvement inquality respiratory performance, which may result in a decrease in PPC risk,23–25 either pneumonia27 or atelectasis,22,27 and reduced LHS.20,22,24 Valkenet et al. (2013), who produced low level of evidence study (observational cohort study), still managed to have two study groups and did not find evidence that preoperative IMT could result in lower rates of PPC or LHS26.
Overall effect of preoperative breathing therapyHere we will present the results of the meta-analysis from all trials using Review Manager 5.3®. We had enough information to analyze the impact on PPC of the preoperative breathing exercises intervention among elderly persons.
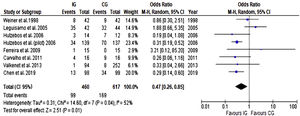
Postoperative pulmonary complicationsBased on data from eight trials (1077 participants), there was a significant reduction in the relative risk of developing PPC with preoperative breathing therapy exercises as presented in Figure 5. When results from trials included in this meta-analysis were pooled, there was moderate heterogeneity, and the pooled risk of developing PPC was 0.47 (CI 95% 0.26 to 0.85).
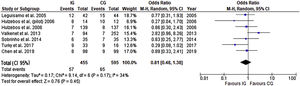
Assessing the benefits of the preoperative intervention in the elderly (65≥years old), by analyzing a subgroup from three of the previous trials (648 participants), there was a significant reduction in the risk of developing PPC (Figure 6). No heterogeneity was present and the risk of developing PPC in this subgroup was 0.30 (CI 95% 0.19 to 0.49).
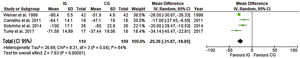
Length of hospital stayData from seven trials (1050 participants) showed a reduction in LHS in the IG with a pooled mean difference of 0.81 days (CI 95% 0.48 to 1.38), with no statistical difference and heterogeneity that might not be significant, as presented in Figure 7.
Postoperative respiratory improvementIn an analysis of seven trials22–26,28,29 (395 participants), heterogeneity was high (I2=96%). Therefore, a subgroup analyses was performed excluding three studies22,23,26 with null CI. Substantially fewer inconsistency were found among the four remaining studies24,25,28,29 (I2=64%).
Thus, preoperative breathing exercise interventions helped improve postoperative ventilation in the analyses of four trials (219 participants), a pooled mean difference of -25.36 (CI 95% -31.87 to -18.85) with substantial heterogeneity (Figure 8).
DiscussionThis systematic review aimed to provide the best available evidence on the effects of a preoperative breathing therapy program on patients undergoing cardiac surgery (CABG and/or valve surgery). The included studies assessed the effectiveness of a breathing therapy program. This intervention could be based solely on breathing exercises or on IMT, using threshold or incentive spirometers, or any of these methods combined.
The results in these studies described the improvement in breathing parameters, assessed pre and postoperatively; the decrease of PPC, such as pneumonia or atelectasis and LHS. Five studies mentioned intensive care unit length of stay and showed no difference between IC and CG.19,23–25,27 Most studies demonstrated that a preoperative breathing intervention is effective at improving respiratory performance after surgery, reducing PPC and LHS.
There was an improvement in inspiratory muscle strength in all studies that established an IMT program through threshold and/or incentive spirometers, which led to better pulmonary function, and may lead to a decrease of PPC risk.19,21,24,25,28,29 This confirms the results obtained by Karanfill & Moller, who determined that preoperative IMT could help reduce the risk of developing PPC.15 However, these authors only studied the impact of IMT, while this review has a wider spectrum, including all types of breathing therapies. Studies that only used breathing therapy also achieved an improvement in the quality of respiratory performance,23 and reduced LHS.22
Participants from all studies were awaiting cardiac surgery; however, most studies only included CABG surgeries,20–25,28,29 three others included CABG and/or valve proceduresç19,26,27 this difference may represent a significant difference in patient recovery. Most studies failed to describe the surgical approach19,20,24,26,27,29; median sternotomy is the only reference to surgical approach in two studies.23,25 Performing a median sternotomy jeopardizes the stability of the thoracic wall; when combined with the removal of the internal mammary artery (IMA), there is a reduction of sanguineous support to the intercostal muscle, leading to a decrease in inspiratory muscle strength.11,30 One support reports that there was no difference in surgical technique between IG and CG; all underwent CABG using IMA, saphenous vein grafting or combined techniques, equally distributed in both groups.28 Hulzebus et al. mentioned the number of affected vessels and number of surgeries requiring cardiopulmonary bypass (CPB), with no statistical difference between CG and IG.21 Leguisamo et al. mentioned that from 86 patients who underwent CABG, 84.9% used the IMA with or without association of a saphenous vein graft and almost 100% of all participants underwent surgery with CPB (no statistical difference between groups); these authors found a high incidence of postoperative pleural effusions (IG – 83.3%; CG – 61.4%) in their studies and equated this with the large number of patients who underwent CABG using the IMA.22 Minimally invasive techniques for classic heart surgery have been developed enabling access to the heart via partial sternotomy for most aortic valve procedures and via sternotomy-free mini-thoracotomy for other procedures, which is leading to a decrease in the overall rate of postoperative complications.31,32 Therefore, it is important for authors to report surgical approach and techniques that may interfere with the research results; we believe this may corroborate the heterogeneity found in meta-analysis. Analysis of surgical pain management postoperatively is important to understand the success of any intervention, because it depends largely on proper pain management during the first few days after a cardiac surgical procedure.33 All included studies failed to report a postoperative pain management protocol.
Patients undergoing heart surgery experienced diminished ventilatory capacity and respiratory muscle strength after surgery.34 Postoperative interventions, such as breathing therapy and early mobilization, may affect results such as a decrease in PPC and LHS due to the reduction in the incidence of atelectasis and pneumonia, especially if patients understand their role in deep breathing and coughing exercise technique to avoid surgical complications.35 Turky et al. had exercises starting one hour after extubation and continued until the eighth postoperative day: patients from the IG practiced deep breathing exercises with threshold as in the preoperative period and were encouraged to cough. On the second day, all patients (IG and CG) were lifted from their beds into a chair and were encouraged to walk short distances. On the third day, patients could walk freely. The CG received routine breathing therapy and early mobilization as described for the IG.25 In the study conducted by Hulzebus et al., both groups underwent incentive spirometry, chest physical therapy and mobilization scheme after surgery.21 Leguisamo et al. (2005) mentioned that conventional physiotherapy was performed twice a day22 and according to Shakouri et al. study participants received physiotherapy based on ward routines.23 Some studies mentioned that both groups received breathing therapy exercises and/early mobilization in the postoperative period, without describing the exercises.19,24,27 Four other studies failed to mention if there were any postoperative breathing or physical interventions.20,26,28,29 Authors, who mentioned the exercises performed postoperatively, stated that both groups had similar approaches after surgery, except for Turky et al. (2017); these authors provided the threshold only for the IG.25 The differences encountered in the postoperative period and the lack of information from most studies may lead to different results between them, contributing to the heterogeneity we encountered.
There is significant statistical heterogeneity in most meta-analysis performed. The variation between study results can be caused by clinical or methodological heterogeneity, wrong choice of treatment effect measures, or at least chance.36 Methodological heterogeneity comprises differences in the design of the included studies - variations related to randomization, allocation secrecy, blinding, losses/exclusions.36As represented in Figure 3, none of the studies managed to blind participants and personnel, except for Weiner et al.;28 Carvalho et al.29 failed to report all design methods. These are examples of unclear differences between study designs that may compromise results. Clinical heterogeneity relates to differences between the study characteristics such as, participant age, surgical technique or postoperative interventions.36 Clinical differences may be due to study typology, if it was an RCT19–26,28,29 or cohort study,27 or number of participants (we included studies that range from 26 participants20 up to 346 participants).27
An additional complexity is that the test for detecting heterogeneity has low power with small sample sizes and few trials are included.37 It is expected that in time, as other studies are performed and included in the review, the results will not be problematic.37 When there is diversity and heterogeneity as encountered in our review, the random effects model is used, which distributes the weight in a more uniform way, valuing the contribution of small studies.36
In the meta-analyses conducted on an older adults subgroup, no heterogeneity was found. This occurred because two studies performed with older participants were conducted by the same researchers and the study designs are similar, one being pilot (weight 9.3%)20 and the final study (weight 83%).21 We decided to keep this analysis, despite its risk of bias, because these studies were performed with elderly participants (age≥70) at high risk of developing PPC, demonstrating the higher value of a preoperative breathing therapy intervention, and a lesser weight study encountered smiliar statistical results27, leading us to believe that this may not be a casualty, although we believe it is necessary to conduct more research with older persons to further check the value of the intervention.
It is well accepted that preadmission interventions, especially in older cardiac surgery patients, may help reduce PPC38,39 and LHS.38 Some studies try to distinguish between the results of each intervention. According to Kehler et al., the literature analyzed does not support the hypothesis that preoperative physical activity is associated with better cardiac surgical outcomes.14 So, does breathing therapy make a difference in postoperative outcomes? Karanfil et al.concluded that IMT, through the use of threshold, decreases the risk of pneumonia and atelectasis.15 But, is it necessary to resort to this device, which may be an added expense and be more time consuming for health professionals? Or is any kind of breathing therapy equally effective? This review seems to support the use of any kind of breathing therapy program that is effective at improving respiratory parameters, and decreasing PPC and LHS, although more studies with greater number of participants are needed.
ConclusionOur findings show that preoperative breathing interventions in patients undergoing cardiac surgery may help improve respiratory performance after surgery, reduce PPC and LHS. However, the heterogeneity encountered may compromise these results. More trials should be conducted to support and strengthen the data found in this systematic review.
Conflicts of interestThe authors have no conflicts of interest to declare.