Aberrant right subclavian artery is the most frequent anomaly of the aortic arch, and in 60% of cases is associated with Kommerell diverticulum. It is usually asymptomatic but may present with dysphagia or asthma due to esophageal and tracheal compression. Indications for surgical repair have not been established; however, when Kommerell diverticulum is complicated by aortic dissection the treatment is surgery. We present the case of a 54-year-old man with thoracic pain due to dissection of an aberrant right subclavian artery associated with Kommerell diverticulum. Elective surgical treatment was performed.
A artéria subclávia direita aberrante é a anomalia mais frequente do arco aórtico, em 60% dos casos é associada ao divertículo de Kommerell. É geralmente assintomática ou pode apresentar disfagia ou dispneia, devido à compressão do esófago ou traqueia. As indicações para a reparação cirúrgica não foram estabelecidas; no entanto, quando o divertículo de Kommerell é complicado por dissecção aórtica, o tratamento é cirúrgico. Apresentamos um caso de um homem de 54 anos, com dor torácica devido à dissecção de uma artéria subclávia direita aberrante associada ao divertículo de Kommerell. É feito um tratamento cirúrgico preferencial.
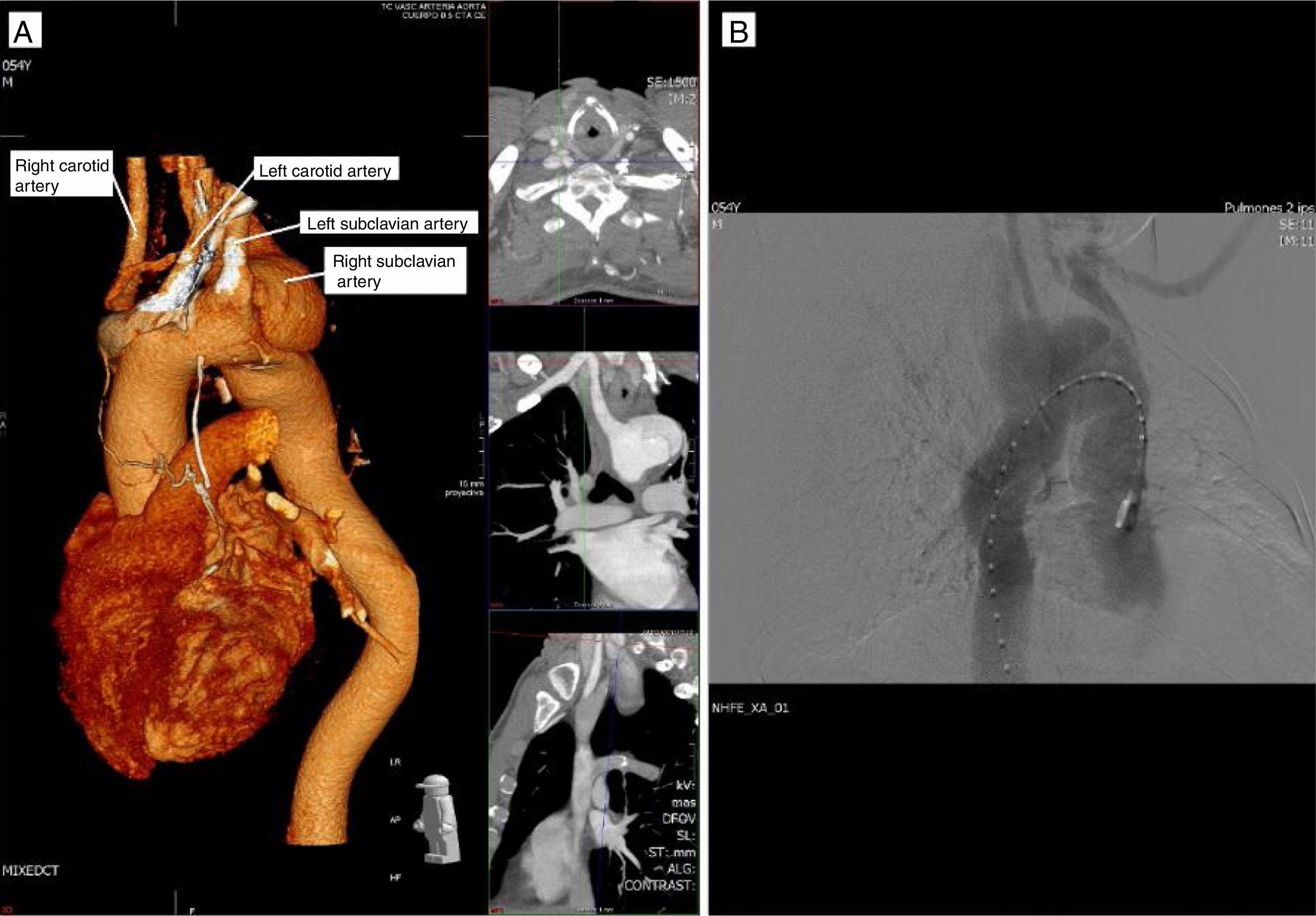
Aberrant right subclavian artery (ARSA) is the most frequent anomaly of the aortic arch, with a reported prevalence ranging between 0.2% and 2.5% of the population,1–3 and is associated with Kommerell diverticulum (KD) in 60% of cases.1,2 KD is one of the vascular ring group of congenital heart defects and consists of a dilatation of the origin of the subclavian arteries.1 It is usually asymptomatic, but when there are symptoms the most frequent is dysphagia due to compression of the esophagus, followed by asthma due to tracheal compression.4 The diagnosis is made by computed tomography angiography (CTA), magnetic resonance imaging, or the reference technique, arteriography.1
When KD is complicated by aortic dissection the treatment is surgery.5 However, in asymptomatic cases there are no well-established guidelines for treatment due to its rarity and heterogeneity of presentation.4 In general, surgical intervention is recommended in symptomatic cases, or when the diverticulum is large (in some cases reaching 50 mm), due to the risk of rupture or embolization.1
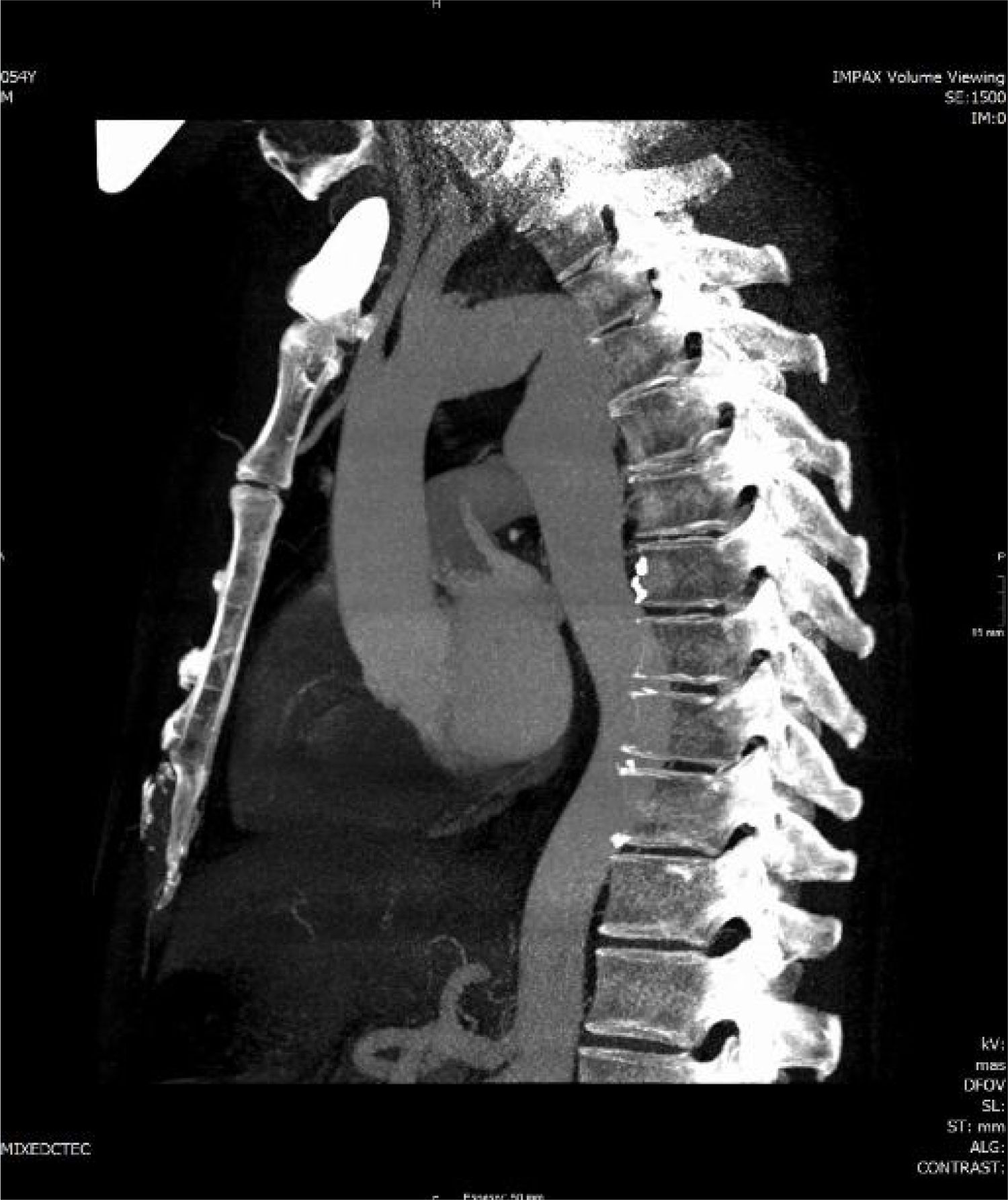
Case reportA 54-year-old man arrived at the hospital with symptoms of abrupt chest pain radiating to the neck, not relieved by nitrates. The patient had a history of ARSA diagnosed in another hospital, with dysphagia of unknown duration. Due to the symptoms of acute aortic syndrome, CTA was performed (Figure 1), findings of which were compatible with a dissecting aneurysm of an ARSA compressing the esophagus. The origin of the left subclavian, left carotid and right carotid arteries were not dissected, nor were the ascending thoracic aorta or the descending thoracic aorta. The maximum diameter of the ascending thoracic aorta was 39 mm. Subsequently, aortography (Figure 1) visualized a bulbous origin of the right subclavian artery, diagnostic of KD. Placement of an endoprosthesis at the outlet of the ARSA was ruled out on technical grounds due to the lack of an anchoring zone between the two subclavian arteries. Echocardiography showed a bicuspid aortic valve without flow alterations, aortic root and ascending aorta of normal size, and both ventricles of normal size and contractility.
Once the patient was stable, elective surgery was performed, through a posterolateral thoracotomy in the fourth left intercostal space. Cardiopulmonary bypass (CPB) was established by femoral artery and vein cannulation. The aortic cross-clamp was placed at the mid-distal arch, proximal to the origin of the left subclavian artery, and on the descending thoracic aorta after the distal margin of the aneurysm. Myocardial arrest was not established, which allowed upper body perfusion with a beating heart through patent supra-aortic trunks. CPB was responsible for lower body perfusion, with the arterial cannula positioned in the descending thoracic aorta; there was no need for deep hypothermic circulatory arrest. The aneurysmal segment was resected and replaced by a 28-mm Dacron tube between the origin of the two subclavian arteries and the descending thoracic aorta distal to the origin of the ARSA. Finally, the proximal segment of the ARSA was ligated with a continuous polypropylene suture in two planes.
The patient's postoperative course was favorable with hemodynamic stability and early extubation. There were no clinical or Doppler signs of critical ischemia in either upper extremity. Postoperative CTA (Figure 2) revealed no sign of complications in the thoracic aorta replacement. Both the right subclavian and the internal thoracic artery were perfused by the vertebral artery through the thoracic inlet. Postoperative echocardiography showed the bicuspid aortic valve to be free of flow alterations, and biventricular function remained good.
Clinically, the patient had dysphonia and dysphagia. At discharge, the dysphagia was resolved but dysphonia persisted. At the six-month postoperative visit, the patient was clinically asymptomatic in cardiorespiratory terms, reporting no chest pain, palpitation or dyspnea.
Preoperative dysphonia is usually due to compression of the recurrent laryngeal nerve, and may be associated with Horner syndrome by compression of sympathetic ganglia and efferent fibers. During surgery, it is important to identify the recurrent nerve as it passes through the aortic arch in order to prevent injury or irritation that may result in dysphonia due to vocal cord paralysis. Once the injury has occurred, vocal rehabilitation may lead to functional recovery, depending on the degree of injury.
DiscussionThe indications for surgical repair in KD have not been established, due to the small number of patients with the condition. Most asymptomatic patients present with rupture. In addition, there are insufficient data to predict rupture based on the size of the diverticulum. However, surgical repair is recommended for symptomatic aneurysms with a diameter of 50 mm or more.6 Our case presented as a complication, and was symptomatic, so surgical repair was recommended.
Some authors recommend surgical repair even in the absence of symptoms due to the risk of aneurysm formation and subsequent dissection, regardless of size at diagnosis. This is related to the embryological origin of this anomaly. In the embryo, the main arterial ducts consist of a right and left dorsal aorta arising from the aortic sac connected to the truncus arteriosus. The six aortic arches give rise to the great arteries. The normal right subclavian artery derives embryologically from the fourth right aortic arch, a portion of the right dorsal aorta and the seventh right intersegmental artery. KD is believed to be a remnant of an isolated residual right dorsal arch.7
KD usually occurs in one of three forms. First, cases with an aberrant left subclavian artery are a consequence of regression in the fourth left aortic arch between the left carotid and the left subclavian arteries. Second, in ARSA, the fourth arch anomaly is the consequence of regression of the right arch between the right subclavian artery and the right and left carotid arteries, and the KD is a remnant of the primitive dorsal right aorta. A third abnormality is a KD in a left aortic arch with a right descending aorta, but this is extremely rare.
Selection of the specific treatment to be adopted is based on patient comorbidities and anatomy, and the surgeon's preference. Less invasive options are preferred as long as the end result is not compromised. Many patients, however, do not have adequate landing zones for fixation of a stent graft even with adjunctive cervical debranching.4 In the case described, there was no area for stent fixation, so we opted for open surgery instead of an endovascular technique. In addition, no replacement of the ascending thoracic aorta was performed because the ascending aorta measured less than 45 mm with a competent bicuspid aortic valve, although patient follow-up was required.
ConclusionARSA is the most frequent anomaly of the aortic arch. In asymptomatic cases there are no well-established guidelines for treatment due to its rarity and heterogeneity of presentation, but when a KD is complicated by aortic dissection the treatment is surgery. Choice of the specific technique used is based on patient comorbidities and anatomy, and the surgeon's preference.
Funding sourcesNo sources of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.