The 8-Item Morisky Medication Adherence Scale (MMAS-8) is one of the most widely used instruments to assess medication adherence, but a validated European Portuguese version of MMAS-8 does not exist. Our aim was to develop and validate a European Portuguese version of the MMAS-8.
MethodsA process of translation and back-translation of the original MMAS-8 was performed. The questionnaire was administered in nine community pharmacies and one public hospital between March 2014 and September 2015. Adult patients taking at least one antihypertensive drug were invited to participate. A confirmatory factor analysis was performed and internal consistency, convergent validity and concurrent validity were examined.
ResultsA total of 472 patients were enrolled in the study. The mean MMAS-8 score obtained was 6.74±1.39. One hundred and thirty-two patients were classified as low adherers (28%), 181 (38.3%) as medium adherers and 159 (33.7%) as high adherers. For the factorial structure of the Portuguese version of the MMAS-8, the fit indices of the final model (chi-square [18] 48.465, p<0.001) are suggestive of very good fit, with comparative fit index 0.95, root mean square error of approximation 0.06 (90% confidence interval 0.04-0.08), and standardized root mean square residual 0.04, confirming that the construct tested was unidimensional. The Cronbach's alpha for all items was 0.60, and the translated version presents convergent validity and concurrent validity.
ConclusionA European Portuguese version of the MMAS-8 was created that maintained a similar structure to the original MMAS-8 and good psychometric properties.
O questionário de adesão 8-Item Morisky Medication Adherence Scale (MMAS-8) é um dos instrumentos mais usados em nível mundial na avaliação da adesão à terapêutica. No entanto, não existe uma versão portuguesa devidamente validada. O nosso objetivo foi desenvolver e validar a versão portuguesa do MMAS-8.
MétodosApós um processo de tradução e retrotradução do MMAS-8 original, a versão portuguesa foi administrada em nove farmácias comunitárias e um hospital público entre março de 2014 e setembro de 2015. Todos os doentes adultos a tomar pelo menos um anti-hipertensor foram convidados a participar no estudo. Foi feita uma análise fatorial confirmatória e foram avaliadas a consistência interna, a validade convergente e a validade concorrente.
ResultadosForam incluídos 472 doentes. A pontuação média obtida no MMAS-8 foi 6,74±1,39. Foram classificados como tendo uma adesão baixa 132 (28%) doentes, 181 (38,3%) como tendo média adesão e 159 (33,7%) como tendo alta adesão. Na avaliação da estrutura fatorial da versão portuguesa do MMAS-8, os índices de ajuste do modelo final (chi-quadrado (18)=48,465, p < 0,001) são sugestivos de um ajuste muito bom, com CFI=0,95; RMSEA=0,06 [intervalo de confiança de 90%: 0,04; 0,08] e SRMR=0,04, e confirmaram que a construção testada é unidimensional. O alfa de Cronbach foi 0,60 e o MMAS-8 apresentou validade convergente e concorrente.
ConclusãoObtivemos uma versão em português do MMAS-8 que manteve uma estrutura semelhante à do MMAS-8 original e com boas propriedades psicométricas.
Low adherence is one of the main causes for therapy failure worldwide.1–3 Self-reported scales are the most commonly used instruments to assess medication adherence in research and in clinical practice.4,5 Considerable efforts have been made in recent years to develop a consistent method to assess medication adherence.
The 8-Item Morisky Medication Adherence Scale (MMAS-8) is probably the most widely accepted self-reported medication adherence instrument.6 Developed in 2008 and based on the 4-Item Morisky Medication Adherence Scale (MMAS-4),7 the MMAS-8 is a unidimensional scale developed to assess adherence in hypertensive patients. The original MMAS-8 version demonstrated high internal consistency (Cronbach's alpha 0.83) and good sensitivity (93%) and specificity (53%) in identifying patients with poor blood pressure control. The MMAS-8 has now been validated in more than 20 languages and for several other diseases.
In Portugal, Delgado et al.8 developed and validated a seven-item questionnaire to assess medication adherence, the Medida de Adesão aos Tratamentos (MAT) (Treatment Adherence Measure), based on the MMAS-47 and Shea et al.9 The MAT is an instrument with good internal consistency (Cronbach's alpha 0.74) that has been used in several studies.10–13 However, it was designed only for use in Portugal, which limits comparability with international studies. So far, there has been no validated European Portuguese version of the MMAS-8. In 2014, a Brazilian Portuguese version of the MMAS-8 was created by Oliveira-Filho et al.14 However, due to significant language differences between Brazilian and European Portuguese, application of the Brazilian Portuguese version in Portugal is not recommended.
Thus, our aim was to develop and validate a European Portuguese version of the MMAS-8 in a Portuguese hypertensive population.
MethodsThis was a cross-sectional study. To obtain a diverse sample of hypertensive patients, data were collected in nine community pharmacies in the central region of Portugal (urban and rural) and in Hospital Infante D. Pedro in Aveiro.
The study was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra (registration number CE_105.2013).
Permission to use the MAT and the Hypertension Knowledge Test (HKT) was kindly granted by their original authors, and a paid license agreement to use the MMAS-8 was established with Donald E. Morisky.
8-item Morisky Medication Adherence ScaleThe instrument consists of seven dichotomous items and a five-point Likert scale. The questions were formulated to avoid a ‘yes-saying’ bias, so one point is assigned to each ‘no’ answer, except in item 5, which is reversed. The eighth question has five possible answers (scores from 0 to 1, in 0.25 point steps). At the end, patients are classified according to the score obtained as low adherers (score <6), medium adherers (score 6 to <8) and high adherers (score 8).6 The original authors also proposed a dichotomous cut-off of low (score <6) vs. high/medium adherence (score ≥6) to facilitate statistical analysis.
Translation and cross-cultural adaptation of the 8-item Morisky Medication Adherence ScaleA process of translation and back-translation was performed according to international guidelines.15,16 Three bilingual translators who were aware of the goals of the study produced three independent translations of the original questionnaire into European Portuguese. The three versions were compared to generate a consensus version. The back-translation, from Portuguese to English, was conducted by another bilingual translator who was not involved in developing the initial version and who was not aware of the objectives of the study. This new English back-translated version was compared to the original version, and a few discrepancies were corrected. Finally, a cross-culturally adapted version was obtained through a consensus meeting attended by two experts in pharmacology and one Portuguese language expert who evaluated its semantic, idiomatic and cultural aspects and conceptual equivalence.
To ensure the instrument's face validity, a pilot test was performed on a Portuguese population (n=20), checking patient understanding and possible doubts and difficulties in the use of the questionnaire. After small adjustments, the final European Portuguese version of the questionnaire was obtained. The patients who participated in the pilot were not included in the following phases of study.
Medida de adesão aos tratamentosThe MAT8 is a validated Portuguese questionnaire developed to assess patients’ adherence to medication. It consists of seven items scaled according to a six-point Likert scale ranging from ‘always’ (1) to ‘never’ (6). The level of adherence is obtained by adding together the points from each item and then dividing by the total number of items. Higher scores are indicative of greater levels of adherence. Patients are classified as adherent or non-adherent according to the median value of the population assessed.
Hypertension Knowledge TestThe HKT17 is a 21-item questionnaire developed to assess patients’ knowledge about hypertension, its causes and treatment, and ways to prevent and control high blood pressure. It is divided into two parts: 12 true/false questions and nine multiple-choice questions. Levels of knowledge are calculated by assigning one point to each correct answer, obtaining a total score that ranges from 0 to 21. The higher the score, the greater the patient's knowledge about hypertension.
Data collectionData were collected between March 2014 and September 2015. Patients were invited to participate if they were over 18 years of age and were taking at least one antihypertensive drug. After informed consent was obtained, a trained pharmacist interviewed the patients in a private office in which data on personal and family history were self-reported, and the MMAS-8, MAT and HKT instruments were administered. The existence of hypertension, stroke, diabetes and dyslipidemia were considered proxies of cardiovascular disease.
Statistical analysisTo examine the factor structure of the European Portuguese version of the MMAS-8, a confirmatory factor analysis (CFA) was performed using AMOS version 20.0 (IBM SPSS AMOS, Meadville, PA, USA). Models were estimated using the maximum likelihood method. Model fit was assessed using the chi-square goodness-of-fit statistic, the comparative fit index (CFI), the standardized root mean square residual (SRMR), and the root mean square error of approximation (RMSEA). Model fit was considered to be very good when the CFI was above 0.95, the SRMR was below 0.10 and the RMSEA was below 0.5.18 Whenever a model did not fit the data well, alternative models were also tested. The development of competing models was based on theoretical considerations and analysis of the data, and two models were tested: one that included all eight items of the MMAS-8 loading on one global factor, overall adherence to antihypertensive medication, and another that included two correlated subscales, one on unintentional non-adherence behaviors with items 1, 2, 4, 5 and 8, and the other on intentional non-adherence behaviors with items 3, 6 and 7.
Internal consistency was examined via Cronbach's alpha, to assess whether various items that set out to measure the same general construct produce similar scores. To assess the correlation between MMAS-8 constructs and other similar adherence measure constructs, convergent validity was assessed by comparing the MMAS-8 and the MAT. Since patients’ knowledge about hypertension has been associated with better adherence levels, concurrent validity was assessed by comparing the MMAS-8 and the HKT, to assess whether the MMAS-8 correlates well with a hypertension knowledge measure.
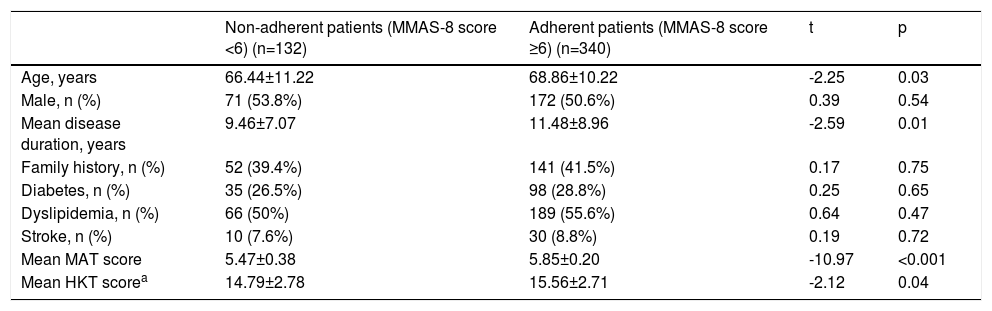
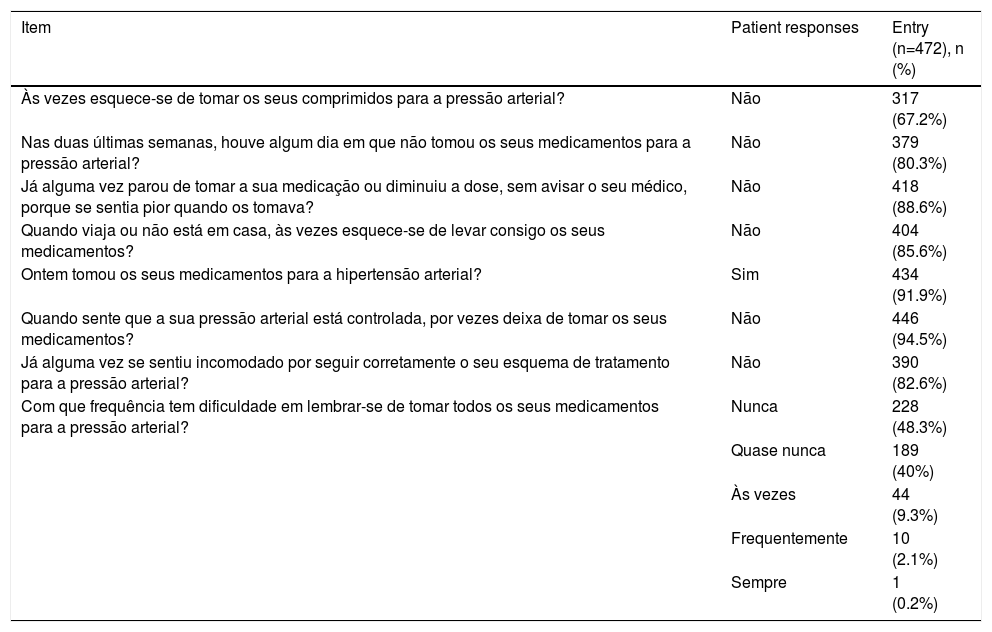
ResultsStudy participantsA total of 472 patients were enrolled in the study, mean age 68.2±10.6 years, 243 (51.5%) female. The mean time since hypertension diagnosis was 10.9±8.5 years, with a maximum disease duration of 50 years. Among the most prevalent comorbidities, 255 (54%) had dyslipidemia, 133 (28%) diabetes, 127 (27%) heart disease, and 40 (8%) had had a stroke. The mean MMAS-8 score obtained was 6.74±1.39. Table 1 presents the characteristics of the study population using dichotomous cut-offs for adherence. Using the three-level cut-offs, 132 (28%), 181 (38.3%) and 159 (33.7%) of patients were in the low, medium and high adherer groups, respectively. Table 2 describes the answers obtained for the MMAS-8. The mean MAT score was 5.74±0.31, and mean HKT score was 15.35±2.79.
Characteristics of the study population according to adherence levels.
| Non-adherent patients (MMAS-8 score <6) (n=132) | Adherent patients (MMAS-8 score ≥6) (n=340) | t | p | |
|---|---|---|---|---|
| Age, years | 66.44±11.22 | 68.86±10.22 | -2.25 | 0.03 |
| Male, n (%) | 71 (53.8%) | 172 (50.6%) | 0.39 | 0.54 |
| Mean disease duration, years | 9.46±7.07 | 11.48±8.96 | -2.59 | 0.01 |
| Family history, n (%) | 52 (39.4%) | 141 (41.5%) | 0.17 | 0.75 |
| Diabetes, n (%) | 35 (26.5%) | 98 (28.8%) | 0.25 | 0.65 |
| Dyslipidemia, n (%) | 66 (50%) | 189 (55.6%) | 0.64 | 0.47 |
| Stroke, n (%) | 10 (7.6%) | 30 (8.8%) | 0.19 | 0.72 |
| Mean MAT score | 5.47±0.38 | 5.85±0.20 | -10.97 | <0.001 |
| Mean HKT scorea | 14.79±2.78 | 15.56±2.71 | -2.12 | 0.04 |
Distribution of responses to individual 8-item Morisky Medication Adherence Scale items.
| Item | Patient responses | Entry (n=472), n (%) |
|---|---|---|
| Às vezes esquece-se de tomar os seus comprimidos para a pressão arterial? | Não | 317 (67.2%) |
| Nas duas últimas semanas, houve algum dia em que não tomou os seus medicamentos para a pressão arterial? | Não | 379 (80.3%) |
| Já alguma vez parou de tomar a sua medicação ou diminuiu a dose, sem avisar o seu médico, porque se sentia pior quando os tomava? | Não | 418 (88.6%) |
| Quando viaja ou não está em casa, às vezes esquece-se de levar consigo os seus medicamentos? | Não | 404 (85.6%) |
| Ontem tomou os seus medicamentos para a hipertensão arterial? | Sim | 434 (91.9%) |
| Quando sente que a sua pressão arterial está controlada, por vezes deixa de tomar os seus medicamentos? | Não | 446 (94.5%) |
| Já alguma vez se sentiu incomodado por seguir corretamente o seu esquema de tratamento para a pressão arterial? | Não | 390 (82.6%) |
| Com que frequência tem dificuldade em lembrar-se de tomar todos os seus medicamentos para a pressão arterial? | Nunca | 228 (48.3%) |
| Quase nunca | 189 (40%) | |
| Às vezes | 44 (9.3%) | |
| Frequentemente | 10 (2.1%) | |
| Sempre | 1 (0.2%) |
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A License agreement is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772; all translations of the MMAS-8 are copyrighted intellectual property and must be obtained from the developer/owner.
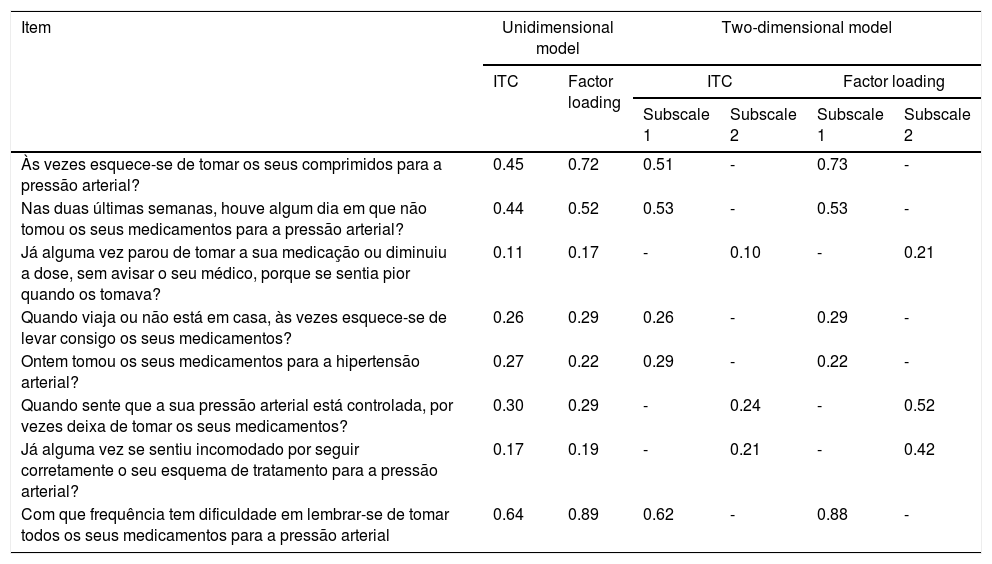
The first CFA model (model 1) included all eight items of the MMAS-8 loading on one global factor, ‘overall adherence to antihypertensive medication’. The model revealed a poor fit to the data, with chi-square 132.13, p<0.001; CFI 0.81; RMSEA 0.11 (90% confidence interval [CI] 0.09-0.13); and SRMR 0.07. Analysis of modification indices and assessment of the phrasing of each item suggested that the introduction of error correlations between items 2 and 5 and between items 6 and 7 would improve model fit, and these were therefore included in the model. The fit indices of this final unidimensional model (chi-square 48.465, p<0.001) suggest a very good fit, with CFI 0.95, RMSEA 0.06 (90% CI 0.04-0.08), and SRMR 0.04.
A second CFA model (model 2) was tested, examining the fit to the data of a theoretical model of two correlated subscales: subscale 1 with items on unintentional non-adherence behaviors and subscale 2 with items on intentional non-adherence behaviors. The same error covariances as in model 1 were introduced in this model. The final two-dimensional model (chi-square [17] 45.90, p<0.001) fit indices suggest a very good fit, with CFI 0.95, RMSEA 0.06 (90% CI 0.04-0.08), and SRMR 0.04.
The differences in chi-square values between the two prior final models revealed that the model fit for the two models was not statistically different (Δ chi-square 1.6, p=0.206).
Internal consistencyThe Cronbach's alpha for all items of a global scale was 0.60; removing any item did not affect alpha significantly. The total correlation coefficient between items for the eight items ranged from 0.11 to 0.64.
Considering the two-dimensional model (model 2), Cronbach's alpha was 0.65 for unintentional non-adherence behaviors and 0.31 for intentional non-adherence behaviors. The total correlation coefficient between items for unintentional non-adherence behaviors ranged from 0.26 to 0.62 and from 0.10 to 0.24 for intentional non-adherence behaviors (Table 3).
Confirmatory factor analysis of the 8-item Morisky Medication Adherence Scale.
| Item | Unidimensional model | Two-dimensional model | ||||
|---|---|---|---|---|---|---|
| ITC | Factor loading | ITC | Factor loading | |||
| Subscale 1 | Subscale 2 | Subscale 1 | Subscale 2 | |||
| Às vezes esquece-se de tomar os seus comprimidos para a pressão arterial? | 0.45 | 0.72 | 0.51 | - | 0.73 | - |
| Nas duas últimas semanas, houve algum dia em que não tomou os seus medicamentos para a pressão arterial? | 0.44 | 0.52 | 0.53 | - | 0.53 | - |
| Já alguma vez parou de tomar a sua medicação ou diminuiu a dose, sem avisar o seu médico, porque se sentia pior quando os tomava? | 0.11 | 0.17 | - | 0.10 | - | 0.21 |
| Quando viaja ou não está em casa, às vezes esquece-se de levar consigo os seus medicamentos? | 0.26 | 0.29 | 0.26 | - | 0.29 | - |
| Ontem tomou os seus medicamentos para a hipertensão arterial? | 0.27 | 0.22 | 0.29 | - | 0.22 | - |
| Quando sente que a sua pressão arterial está controlada, por vezes deixa de tomar os seus medicamentos? | 0.30 | 0.29 | - | 0.24 | - | 0.52 |
| Já alguma vez se sentiu incomodado por seguir corretamente o seu esquema de tratamento para a pressão arterial? | 0.17 | 0.19 | - | 0.21 | - | 0.42 |
| Com que frequência tem dificuldade em lembrar-se de tomar todos os seus medicamentos para a pressão arterial | 0.64 | 0.89 | 0.62 | - | 0.88 | - |
The unidimensional model has Cronbach's alpha 0.60; in the multidimensional model, subscale 1 has Cronbach's alpha 0.65 and subscale 2 has Cronbach's alpha 0.31.
ITC: item total correlation coefficient.
Use of the ©MMAS is protected by US copyright laws. Permission for use is required. A License agreement is available from Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772; all translations of the MMAS-8 are copyrighted intellectual property and must be obtained from the developer/owner.
The MMAS-8 was highly and significantly correlated with the MAT (0.67, p<0.001), confirming that both instruments are assessing correlated constructs and have good concurrent validity.
The MMAS-8 was significantly correlated with the HKT (0.14, p=0.014), demonstrating a high convergent validity. Using Morisky et al.’s dichotomous classifications of patients as adherent with MMAS-8 score ≥6 and non-adherent with MMAS-8 score <6,6 there were significant differences in the HKT score between the two groups (t[295] 2.123, p=0.035). Adherent participants had greater knowledge about hypertension than non-adherent patients (HKT score 15.56±2.77 and 14.79±2.78, respectively).
DiscussionWe developed and validated a European Portuguese version of the MMAS-8 in a sample of hypertensive patients, using CFA to examine its factorial structure. The result was a version with an acceptable internal consistency, albeit considerably lower than the original version (Cronbach's alpha 0.60 vs. 0.83, respectively).6 Similar problems regarding internal consistency have arisen in other MMAS-8 validation studies, such as the French version (alpha 0.54)19 and the Korean version (alpha 0.56),20 both also using samples of hypertensive patients. Other studies using patients with different medical conditions presented even lower internal consistency, such as the German validation with patients under chronic antiplatelet treatment (alpha 0.31),21 the Chinese validation with patients suffering epilepsy (alpha 0.56),22 and the Thai validation with diabetic patients (alpha 0.61).23
One of the explanations for this low internal consistency given by some of the authors of these validation studies was the potential multidimensionality of the MMAS-8, rather than simple inconsistency.20,21,23 To check this possible multidimensionality, and considering the differences between intentional and unintentional non-adherence, we tested a two-dimensional model for the MMAS-8. In the two-dimensional model, internal consistency was also below the recommended cut-off of 0.65. No significant differences were found between the original unidimensional model and the two-dimensional model, suggesting that both models could be used interchangeably.
Voils et al.24 suggested that conflation of cause and effect indicators could cause the low internal consistency of self-reported adherence instruments. Because non-adherence can be affected by a multiplicity of factors, including patient-related, socioeconomic and health system-related factors,25 low correlation between these factors may be expected. As such, the value of statistics such as Cronbach's alpha depends upon high inter-item correlation, and they may be inappropriate if used as a single indicator of a multidimensional scale.
As with validation studies of the original MMAS-86 and other versions,20,21,26 the European Portuguese version of the MMAS-8 presented good concurrent and convergent validity. Cross-cultural adaptation has thus produced a valid and reliable European Portuguese version of the MMAS-8.
Study limitationsThe selection of a sample of hypertensive patients may be a limitation of this study, preventing generalization to other conditions.
ConclusionWe developed a European Portuguese version of the MMAS-8 that presented acceptable internal consistency and good convergent and concurrent validity; this version can be used in research and in clinical practice. Further investigation is required to assess its psychometric properties in other medical conditions.
Acknowledgments and disclosuresThe authors wish to thank DE Morisky, who gave permission for use of the MMAS-8 for this study. The use of the ©MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, Los Angeles, CA 90095-1772; all translations of the MMAS-8 are copyrighted intellectual property and must be obtained from the developer/owner.
Conflicts of interestThe authors have no conflicts of interest to declare.