Endomyocardial biopsy is still the principal method for diagnosing cardiac allograft rejection. However, this procedure can be associated, albeit rarely, with potentially serious complications.
We describe the case of a patient with extensive anterior myocardial infarction without revascularization, who developed cardiogenic shock and required heart transplantation. Post-transplantation, a coronary artery fistula to the right ventricle associated with an aneurysm and two restrictive interventricular communications were detected.
A biópsia endomiocárdica continua a ser o principal método de monitorização da rejeição em recetores de transplante cardíaco. No entanto, este procedimento pode estar associado, ainda que raramente, a complicações potencialmente graves.
Descreve-se o caso de um doente com enfarte anterior extenso não revascularizado, com evolução em choque cardiogénico e necessidade de transplante cardíaco. Na fase pós-transplante é detetada fístula coronária para o ventrículo direito com aneurisma associado e duas comunicações interventriculares restritivas.
Endomyocardial biopsy is still the gold standard method to screen for rejection in orthotopic heart transplant recipients. It should be performed weekly in the first month and regularly during the first year after transplatation.1 Complications related to the procedure have been reported, although they are rare and usually without long-term sequelae.2 The most common are related to vascular access, but allergic reactions, cardiac perforation with tamponade, embolism, arrhythmias,3 conduction disturbances, damage to the tricuspid valve apparatus with regurgitation,4 and coronary fistulas5–7 have also been described. Based on a case of coronary fistula to the right ventricle associated with an aneurysm and two restrictive interventricular communications in a heart transplant patient following multiple endomyocardial biopsies, we discuss the various therapeutic options available and question the systematic use of endomyocardial biopsy to detect allograft rejection.
Case reportA 52-year-old man, with hypertension, dyslipidemia, smoking and a family history of coronary artery disease as cardiovascular risk factors, was admitted to our department in April 2008 for anterior myocardial infarction of two days’ evolution, without revascularization. On admission, the patient presented no murmur on cardiac auscultation; pulmonary auscultation revealed bibasal rales. His blood pressure was 105/89mmHg and heart rate was 122bpm. The electrocardiogram showed sinus tachycardia, incomplete right bundle branch block and Q waves in V1 to V4 and aVL, indicating an anterior infarction scar. Transthoracic echocardiography revealed left ventricular apical akinesia and hypokinesia of the entire anterior, lateral and interventricular septal territory, causing severe global systolic dysfunction. Coronary angiography performed on the first day of hospitalization revealed a dominant trifurcated left main and an occluded left anterior descending (LAD) ostium with no visible rim, with retrograde filling of a short distal segment. In view of the time since symptom onset, coronary anatomy and the clinical situation of incipient heart failure, it was decided to insert an intra-aortic balloon pump. Myocardial perfusion scintigraphy showed the entire LAD territory to be predominantly non-viable due to severe necrosis, and so cardiac magnetic resonance imaging was suggested. However, on the 10th day of hospitalization, the patient suffered cardiopulmonary arrest in ventricular tachycardia. He was resuscitated and ventilated, intravenous inotropic therapy was begun and a Biomedics™ left ventricular assist device was implanted, later replaced by a Berlin Heart™ type device. The patient then developed cardiogenic shock with multiple episodes of malignant arrhythmia requiring a variety of antiarrhythmic drugs (amiodarone, lidocaine and esmolol). His stay in the intensive care unit was complicated by septic shock, Candida albicans esophagitis, hemolytic anemia and an episode of acute ischemia due to embolization to the common femoral artery, treated by embolectomy. Subsequent pathoanatomical study revealed a thrombus containing fibrin, erythrocytes and granulocytes with Aspergillus, and antifungal therapy was begun with voriconazole.
The patient remained under ventricular assistance for 135 days and he was transplanted in September 2008. The postoperative course was uneventful and he was discharged after two months, under triple immunosuppressive therapy (tacrolimus, mycophenolate mofetil and prednisolone), together with verapamil 180mg, bisoprolol 2.5mg and pravastatin. Thirteen endomyocardial biopsies were performed during the first 24 months.
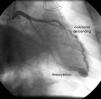
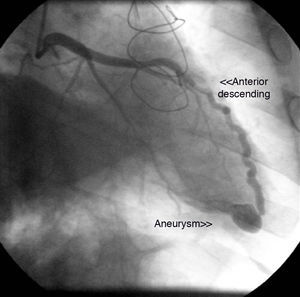
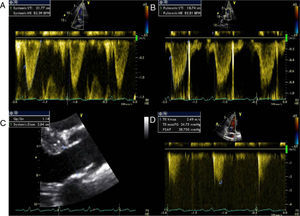
The first coronary angiogram performed two years after transplantation detected an image suggestive of a small fistula from the distal LAD to the right ventricle, with an associated aneurysm (Figure 1). Repeat myocardial perfusion scintigraphy excluded ischemia and showed absence of left ventricular dilatation and normal systolic function. Repeat right and left heart catheterization 15 days later revealed pulmonary artery systolic pressure of 32mmHg, right atrial pressure of 10mmHg, and elevated systemic cardiac output (9.2l/min; cardiac index 4.8l/min/m2). No fall in pulmonary artery pressure was observed following occlusion of the fistula with a 4/9mm balloon and no significant shunt was detected; Qp/Qs ratio was 1.39.
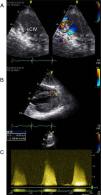
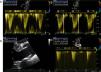
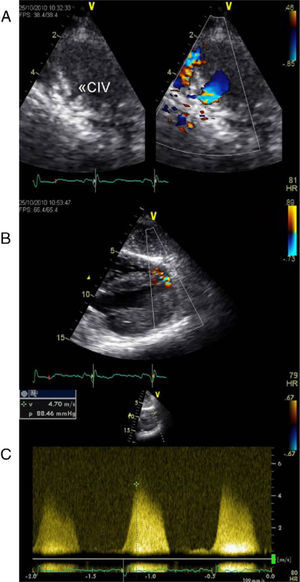
Echocardiographic assessment revealed persistence of two restrictive interventricular communications in the apical and mid segments of the ventricular septum (Figure 2) but no significant shunt (Figure 3). Since the patient had no symptoms of heart failure or angina, it was decided to adopt a conservative strategy.
Transthoracic echocardiogram two years after heart transplantation showing two solutions of continuity (A and B) with two high-velocity jets (maximum 5m/s) (C) from the left to the right ventricle, located in the apical and mid segments of the interventricular septum, that appear to be two restrictive interventricular communications. CIV: interventricular communication.
Coronary fistulas are a rare cardiac anomaly, whose incidence is thought to be less than 0.2%, although it is reported to be higher in heart transplant patients (5–8%).6,7 They can be congenital or acquired. In the case presented, the location of the fistula from the distal LAD to the right ventricle suggests an acquired cause, probably the result of trauma during multiple biopsies. The simultaneous presence of two small, restrictive interventricular communications could suggest that both anomalies were acquired. However, the coexistence of such anomalies in a transplant recipient has not been described in the literature and the possibility that they already existed in the donor heart cannot be excluded.
Coronary angiography is still the gold standard exam for diagnosis and hemodynamic assessment of coronary fistulas, and in this case it excluded any significant repercussions of either the fistula or the interventricular communications. The shunts resulting from these two entities were small and not associated with symptoms. In fact, most coronary fistulas are detected incidentally during the first coronary angiography following heart transplantation.8 The natural history of coronary fistulas arising from endomyocardial biopsies in heart transplant patients is usually benign,9 and in most cases a conservative approach is adopted. Elective closure may be performed if the fistula is associated with a hemodynamically significant shunt or symptoms, particularly heart failure, ischemia due to myocardial steal, thrombosis and embolization, arrhythmias or endocarditis.9 When closure is indicated, therapeutic options include surgery and percutaneous intervention.9,10 In recent years, percutaneous closure has been gaining ground, using embolization products such as balloons or coils,11 and more recently Amplatzer devices.12 In the case presented, the patient was asymptomatic, with no evidence of ischemia on myocardial perfusion scintigraphy, and so a conservative approach was deemed the best strategy. Nevertheless, due to the increased risk of endocarditis, prophylactic therapy is mandatory.13 There are no specific guidelines on follow-up in these situations, but angiographic assessment is probably justified to monitor progressive proximal dilatation of the coronary artery involved.
As described above, endomyocardial biopsy is not without risks, and it is therefore worth considering new non-invasive methods based on gene-expression profiling, particularly in patients at low risk of rejection.14
The present case also illustrates the malignant course of an extensive anterior myocardial infarction without revascularization. It is nevertheless a case of survival, and highlights the importance of early institution of mechanical circulatory support as a bridge to heart transplantation. Although routine myocardial biopsy following heart transplantation is an invasive screening method that causes the patient discomfort, its possible complications are a lesser evil and it continues to be the principal method for diagnosing allograft rejection.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Dr. Maria João Andrade and other staff in the echocardiography Department of Hospital de Santa Cruz for their help in acquiring the images.
Please cite this article as: Calé R, et al. Complicações da biópsia endomiocárdica após transplante cardíaco. Um mal menor. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2011.12.006.