Atrial fibrillation is a supraventricular arrhythmia that increases the risk of ischemic stroke and other thromboembolic events. Recently new treatment options have emerged whose cost-effectiveness relative to conventional therapy (warfarin) is well demonstrated. This study compares the clinical benefits and economic costs associated with the new oral anticoagulants most used in Portugal: dabigatran and rivaroxaban.
MethodsThe results of an indirect comparison of the RE-LY and ROCKET AF trials, which enabled differences in the efficacy of dabigatran and rivaroxaban to be determined, were used in a Markov model simulating patient outcomes in terms of ischemic and hemorrhagic stroke, transient ischemic attack, systemic embolism, acute myocardial infarction and intra- and extracranial bleeding.
ResultsThe use of dabigatran is associated with better clinical results. The reduction in events is reflected in longer survival (8.41 vs. 8.26 years) and more quality-adjusted life years (5.87 vs. 5.74), while the lower daily treatment cost and the reduction in event-related costs lead to a saving of 367 euros per patient from a societal perspective.
ConclusionsThe results show that dabigatran is a dominant alternative, i.e., it produces better clinical results at a lower cost. Sensitivity analysis demonstrates that the results are robust even considering the uncertainty inherent in an indirect comparison. It can thus be concluded that in clinical practice in Portugal the use of dabigatran is to be preferred to the use of rivaroxaban.
A fibrilhação auricular é uma arritmia supraventricular que aumenta o risco de acidentes vasculares cerebrais isquémicos e de outros eventos tromboembólicos. Recentemente surgiram novas opções cuja custo-efetividade relativamente à terapêutica tradicional – varfarina – está bem demonstrada. Neste estudo comparam-se os benefícios clínicos e os custos económicos associados às duas novas opções mais utilizadas em Portugal: dabigatrano e rivaroxabano.
MétodosOs resultados de uma comparação indireta dos ensaios RE-LY e ROCKET AF, que permitiu determinar diferenças de eficácia entre dabigatrano e rivaroxabano, foram utilizados num modelo de Markov que simula a evolução dos doentes prevendo a ocorrência de acidentes vasculares cerebrais isquémicos e hemorrágicos, de acidentes isquémicos transitórios, de embolias sistémicas, de enfartes agudos do miocárdio e de hemorragias intra e extracranianas.
ResultadosA utilização de dabigatrano está associada a melhores resultados clínicos. De facto, a diminuição de eventos reflete-se numa maior sobrevida (8,41 versus 8,26 anos) e em mais anos de vida ajustados pela qualidade (5,87 versus 5,74). Paralelamente, o menor custo diário de tratamento e a redução de custos com eventos conduzem a uma poupança de recursos valorizada em 367 € por doente, na perspetiva da sociedade.
ConclusõesOs resultados mostram que o dabigatrano constitui uma alternativa dominante, ou seja, permite obter melhores resultados clínicos com menores custos. A análise de sensibilidade demonstra que os resultados são robustos, mesmo quando considerada a incerteza inerente a uma comparação indireta. Assim, é possível concluir que na prática clínica portuguesa a utilização de dabigatrano deve ser preferida à utilização de rivaroxabano.
atrial fibrillation
confidence interval
hazard ratio
intracranial hemorrhage
international normalized ratio
myocardial infarction
quality-adjusted life years
relative risk
systemic embolism
transient ischemic attack
time in therapeutic range
value-added tax
Atrial fibrillation (AF) is a supraventricular arrhythmia characterized by uncoordinated atrial activation with consequent deterioration of atrial mechanical function. Patients with AF are therefore at increased risk of thromboembolic events, particularly ischemic stroke. Furthermore, as well as a three- to four-fold increase in incidence, the effects of ischemic stroke resulting from AF tend to be especially severe, causing death or disability in 80% of cases and one-year mortality of around 50%.1,2 Prevention of such events is thus the main aim of oral anticoagulation in AF patients. However, it is essential to ensure that this therapy does not increase the incidence of intracranial hemorrhage (ICH), which in most cases is fatal.3
According to the FAMA study, the overall prevalence of AF in Portugal is 2.5% in those aged over 40, with a clear relationship with age: 0.2% in those aged 40–49, 1.0% in those aged 50–59, 1.6% in those aged 60–69, 6.6% in those aged 70–79 and 10.4% in those aged 80 or more.4 It can be assumed that this relationship means that an aging population will lead to an increase in overall prevalence. At the same time, according to the Euro Heart Survey,5 only 62% of individuals with AF are aware of the condition, possibly because it is frequently asymptomatic.
For nearly 50 years, the main preventive therapy available was oral anticoagulation in the form of vitamin K antagonists, of which warfarin is the most widely used. Studies show that oral anticoagulants reduce ischemic stroke risk by 64% compared to placebo and by 38% compared to aspirin.6 However, since warfarin's efficacy and safety depend on controlling the international normalized ratio (INR), which is affected by diet and drug interactions, a significant proportion of eligible patients (estimated at 62% in Portugal4) are not medicated with warfarin.
Dabigatran is a reversible direct thrombin inhibitor that has been approved for prevention of stroke and systemic embolism (SE) in AF patients indicated for oral anticoagulation. In the RE-LY trial, in a direct comparison with warfarin in over 18000 patients, dabigatran significantly reduced stroke and SE at a dose of 150 mg twice daily, with relative risk (RR) of 0.66, 95% confidence interval (CI) 0.53–0.82 (p<0.001 for superiority). The 110 mg twice daily dose was not inferior to dose-adjusted warfarin in preventing stroke and SE, with reduced incidence of bleeding in both treatment arms.7,8 On the basis of these results, the European Medicines Agency recommended the use of 110 mg twice daily for those aged 80 or above, those at increased risk of bleeding, and those also taking verapamil, and 150 mg twice daily for the remainder.9 Re-analysis of the RE-LY results according to this age division showed that the RR of ischemic stroke with dabigatran is 0.77 for those aged under 80 and 0.82 for older patients, while RRs for SE were 0.66 and 0.51, respectively. It also demonstrated that dabigatran reduced ICH by more than 50% in all age-groups, with RRs of 0.48 for those aged under 80 and 0.29 for those aged over 80, while there was increased extracranial bleeding in the older age-group (RR 1.44). On the basis of these results the 2012 update of the European Society of Cardiology guidelines recommend dabigatran for AF patients at moderate to high risk of events.10
Rivaroxaban, a direct factor Xa inhibitor, is also approved for prevention of stroke and SE in AF patients indicated for oral anticoagulation. The ROCKET AF trial, with around 14000 patients, showed that 20 mg rivaroxaban is not inferior to warfarin in preventing stroke and SE (hazard ratio [HR] 0.88; 95% CI 0.75–1.03; p<0.001 for non-inferiority; p=0.12 for superiority) and reduced ICH (HR 0.67), but was associated with increased major bleeding (HR 1.04).11
Previous studies have estimated the cost-effectiveness of dabigatran12 and rivaroxaban13 compared to the alternatives available at the time (warfarin, aspirin and no treatment). However, it is important to compare the clinical benefits and economic costs associated with each drug in clinical practice in Portugal in a single study. This will assist healthcare providers in their choice between the two new oral anticoagulants most used in Portugal.14
Direct comparison between the RE-LY and ROCKET AF trials is difficult, due to differences in study population, design, and primary analysis. ROCKET AF included patients at higher stroke risk as demonstrated by the mean CHADS2 scores (2.1 in RE-LY vs. 3.5 in ROCKET AF). Furthermore, patients in the warfarin arm of RE-LY had a better time in therapeutic range (TTR) than in ROCKET AF (64.4% vs. 55.2%, respectively).
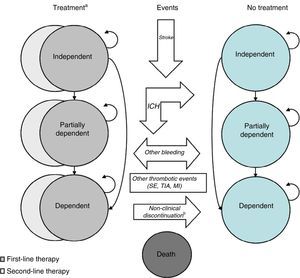
MethodsModel descriptionThe Markov model used in this study simulates the occurrence of the most important clinical events in the context of AF: death, ischemic stroke, transient ischemic attack (TIA), SE, myocardial infarction (MI), ICH (including hemorrhagic stroke), extracranial bleeding, and minor bleeding. The model assumes that only one event can occur in any three-month cycle, with the exception of minor bleeding, which can occur in the same period as another event.
Cerebrovascular events (ICH and ischemic stroke) are assumed to cause loss of independence (leading to partial or complete dependence). ICH is also assumed to result in permanent discontinuation of oral anticoagulation therapy, while treatment may be suspended temporarily due to extracranial bleeding or other reasons. In either case, treatment discontinuation is assumed to lead to preventive treatment with aspirin.
Since the model simulates the evolution of the cohort until death, it enables estimation of costs, life years and quality-adjusted life years (QALYs) associated with the alternatives being compared, dabigatran and rivaroxaban. It is thus possible to calculate the incremental cost per QALY and per life year gained.
The model (presented in Figure 1) was first developed by the University of British Columbia to compare dabigatran, warfarin, aspirin and no treatment. It was originally published for the Canadian context,15 and has been adapted for other countries, including the UK16 and Portugal.12
Clinical dataThe clinical data used in this analysis are a combination of the results of the RE-LY and ROCKET AF trials, in which warfarin adjusted for a target international normalized ratio (INR) between 2.0 and 3.0 was compared with dabigatran (110 mg twice daily or 150 mg twice daily) and rivaroxaban (20 mg daily), respectively.
The existence of a common comparator in the two trials means that indirect methods can be used to compare the results of treatment with dabigatran and rivaroxaban.1 When performing indirect comparisons, the results of the trials usually need to be adjusted in order to account for differences in the characteristics of the patient cohorts that could affect outcomes, particularly event rates. In the present case, it is also necessary to consider the success of keeping INR within the target therapeutic range, since this is essential for both the efficacy and safety of warfarin therapy.
An indirect comparison was accordingly performed19 in which the event rates reflect the characteristics of the ROCKET AF study population, who had higher thromboembolic risk than that of RE-LY as indicated by CHADS2 scores.
With regard to INR control, the results in RE-LY were better, with a mean TTR of 64.4% compared to 55.2% in ROCKET AF. However, since demographic differences between the cohorts affected patients’ ability to keep INR within the therapeutic range, the indirect comparison used a study by the US Food and Drug Administration which concluded that if the ROCKET AF study population had been similar to that of RE-LY, its mean TTR would have been 57.47%.20
The conclusions of the indirect comparison are described in Table 1, which shows that, compared to dabigatran, rivaroxaban is associated with 40% higher risk for ischemic stroke, 166% higher risk for ICH, and 26% higher risk for extracranial bleeding. However, risk for MI and SE is 35% and 50% lower, respectively.2
Event rates per 100 person-years for those taking dabigatran and relative risks compared to rivaroxaban and aspirin.
The reduction in event rates resulting from the treatments under analysis not only increases patients’ survival, but also improves quality of life. Stroke, for example, affects both the individual's prognosis and their immediate quality of life. It is therefore important to include quality-of-life weights in an economic evaluation in order to calculate QALYs. In the present study, we used the results of a meta-analysis21 of 20 studies measuring quality of life following stroke, on the basis of which quality-of-life weights were established for each level of dependence, and of another study that assessed the impact of other events.22 The weights used are presented in Table 2.
Quality-of-life weights.
| Quality-of-life weight by level of dependence | |
| Independent, no history of stroke | 0.81 |
| Independent | 0.65 |
| Dependent | 0.46 |
| Totally dependent | 0.30 |
| Reduction of quality of life associated with each event | |
| Stroke | 0.139 |
| SE | 0.120 |
| TIA | 0.103 |
| ICH | 0.181 |
| Extracranial bleeding | 0.181 |
| Minor bleeding | 0.004 |
| MI | 0.125 |
ICH: intracranial bleeding; MI: myocardial infarction; SE: systemic embolism; TIA: transient ischemic attack.
The economic data used are those presented in a previous cost-effectiveness evaluation comparing dabigatran with warfarin, aspirin and no treatment.12 In that study, the resources consumed for treatment and follow-up of events were estimated on the basis of information provided by a panel of experts composed of six specialists with proven clinical experience in following such patients.
The costs of following patients and treating acute events were calculated as the combination of the resources identified by the expert panel and the respective unit costs, excluding value added tax (VAT)3 (Table 3). The daily costs of the treatments being compared (also excluding VAT) are currently 2.36 euros for dabigatran 110 mg twice daily, 2.46 euros for dabigatran 150 mg twice daily, and 2.47 euros for rivaroxaban 20 mg daily.
Economic data (in euros).
| Daily cost of treatment | |
| Dabigatran 150 mg twice daily | 2.46 |
| Dabigatran 110 mg twice daily | 2.36 |
| Rivaroxaban 20 mg daily | 2.47 |
| Aspirin 150 mg once daily | 0.09 |
| Cost per event (acute phase) | |
| Ischemic or hemorrhagic stroke | 4094.47 |
| SE | 1522.15 |
| TIA | 3183.60 |
| ICH | 5158.85 |
| Fatal extracranial bleeding | 1764.71 |
| Extracranial bleeding | 1445.40 |
| Fatal MI | 3153.49 |
| MI | 3077.06 |
| Three-monthly cost of follow-up | |
| No event | 96.93 |
| Independent | 121.48 |
| Partially dependent | 152.02 |
| Completely dependent | 2879.99 |
| Three-monthly cost of rehabilitation following ischemic or hemorrhagic stroke or ICH | |
| Independent | |
| First year | 82.50 |
| Partially dependent | |
| First three months | 2515.06 |
| Remainder of first year | 1333.00 |
| Following years | 283.50 |
| Dependent | |
| First three months | 2337.94 |
| Remainder of first year | 1155.88 |
ICH: intracranial bleeding; MI: myocardial infarction; SE: systemic embolism; TIA: transient ischemic attack.
The resources consumed were calculated from a societal perspective, considering overall costs, whether paid by the State or by patients.
ResultsBase scenarioThe most important finding is the demonstration that for the prevention of thromboembolic events in AF patients, dabigatran gives better clinical results than rivaroxaban. The model predicts that patients taking dabigatran will suffer less ischemic stroke (0.27 vs. 0.31), ICH (0.03 vs. 0.06), and extracranial bleeding (0.24 vs. 0.28), but more MI (0.17 vs. 0.13) and SE (0.024 vs. 0.021).
As stated above, the occurrence of events affects both prognosis and quality of life, and the reduction in events with dabigatran means patients have longer survival (8.41 vs. 8.26 years) and more QALYs (5.87 vs. 5.74).
Dabigatran is also superior in economic terms, since the lower event rates result in reduced consumption of resources in follow-up and treatment of acute episodes (Table 4). However, despite its lower daily treatment cost, the longer survival with dabigatran means that the total treatment cost is higher. Over a lifetime, preventive therapy with dabigatran saves 367 euros per patient, calculated as an increased treatment cost of 150 euros and a reduction of 518 euros in the cost of treatment of events and follow-up. Dabigatran is thus the dominant option, being both more effective and less costly. Detailed results are presented in Table 4.
Sensitivity analysisAs in all economic evaluations, the results presented in the base scenario are affected by underlying assumptions. It is thus essential to perform a sensitivity analysis to assess to what extent the results depend on these assumptions.
For some parameters, such as the likelihood of events occurring, for which the uncertainty can be characterized by a statistical distribution, a probabilistic sensitivity analysis can simultaneously assess the impact of different occurrence rates of each parameter. As well as enabling simultaneous analysis of uncertainties, this method can also calculate the proportion of simulations in which the cost-effectiveness ratio is less than the established willingness to pay threshold.
However, for other parameters such as discount rate and time horizon, a univariate sensitivity analysis is usually performed to assess the impact of assuming alternative values, since the uncertainty of such parameters cannot be associated with a statistical distribution. The discount rate (5%) and the time horizon (lifetime) used in the base scenario are those stipulated in the methodological guidelines in force in Portugal.23
Although the uncertainty regarding costs could theoretically be characterized by a statistical distribution, the fact that they were obtained from an expert panel decreases the robustness of this procedure. It was therefore decided to carry out a univariate sensitivity analysis, the results of which are described in Table 5, showing that dabigatran is dominant in all scenarios. The probabilistic sensitivity analysis (Figure 2) confirms this, with 99.8% of the 1000 simulations revealing dabigatran as dominant.
Univariate sensitivity analysis.
| Base scenario | Discount rate | Time horizon | Costs | ||||
|---|---|---|---|---|---|---|---|
| 3% | 7% | 10 years | 15 years | +20% | −20% | ||
| Incremental life years | 0.14 | 0.18 | 0.12 | 0.07 | 0.11 | 0.14 | 0.14 |
| Incremental QALYs | 0.13 | 0.16 | 0.11 | 0.07 | 0.11 | 0.13 | 0.13 |
| Incremental costs (in euros) | −367 | −411 | −330 | −310 | −372 | −471 | −264 |
QALYs: quality-adjusted life years.
Since there have been no trials directly comparing dabigatran and rivaroxaban for patients with non-valvular AF, the only way to compare them is indirectly, simulating the clinical effects of the two drugs in equivalent situations.
This type of analysis is naturally subject to greater uncertainty, which is reflected in the number of relative risk values presented in Table 1 that do not reach statistical significance. However, indirect comparisons are essential if health care providers are to make informed and evidence-based choices between alternatives. Merely comparing the relative risks reported in the RE-LY and ROCKET AF trials would disregard not only the baseline difference between the study cohorts but also, more importantly, the fact that the mean TTR of patients taking warfarin in the two trials was substantially different, which reduces the efficacy and safety of warfarin in ROCKET AF and leads to the relative risks of rivaroxaban therapy being better than they would have been if the TTR in that trial had been greater.
Furthermore, several health authorities have incorporated the results of the economic evaluation model used in their reimbursement decisions, which supports the model's robustness. The results are also consistent with those obtained in an independent evaluation performed under the aegis of the Canadian Agency for Drugs and Technologies in Health.24
The results of the present study appear to contradict those in economic evaluations in which dabigatran12 and rivaroxaban13 were compared with a combined comparator of warfarin, aspirin and no treatment. The cost per QALY obtained with rivaroxaban (6697 euros) was lower than that reported for dabigatran (8409 euros). However, this is due to considerable differences in the costs of monitoring INR; if the same monitoring costs (those for rivaroxaban) are used in both studies, the cost per QALY for dabigatran is 5108 euros compared with the combined comparator, making dabigatran dominant, just as when the comparison is with warfarin only. Finally, the fact that costs arising from stroke are lower in Portugal than in other countries for which similar economic evaluations have been published,15,16 as well as in most of those included in the statistics published by the European Heath Network,25 reduces the advantage of dabigatran, since one of the main benefits of the drug is in reducing the incidence of stroke, which is the event with the greatest clinical and economic impact.
ConclusionsThe results of this economic evaluation show that dabigatran is better than rivaroxaban for preventing thromboembolic events in patients with non-valvular AF. This is due to the lower incidence of ischemic stroke and ICH and their long-term consequences.
Overall, the clinical gains are longer survival (0.14 years) and more QALYs (0.13). In economic terms, the results show that dabigatran also leads to savings, making it a dominant alternative, being both more effective and less costly. In addition, the univariate sensitivity analysis demonstrates the robustness of the results, with dabigatran dominant in all scenarios. Even in the probabilistic sensitivity analysis (which incorporates the uncertainty associated with estimation of relative risks) dabigatran was dominant in almost all simulations.
It can thus be concluded that in clinical practice in Portugal, dabigatran is a better option than rivaroxaban for prevention of thromboembolic events in patients with non-valvular AF.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingFunding for this study was provided by Boehringer Ingelheim, Lda., and was not dependent on obtaining any specific results.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Miguel LS, Ferreira J. Consequências clínicas e económicas da utilização de dabigatrano e de rivaroxabano em doentes com fibrilhação auricular não valvular. Rev Port Cardiol. 2016;35:141–148.
Recognition of the value of indirect comparisons, together with the need to ensure good research practice, led to the establishment of the Task Force on Indirect Treatment Comparisons of the International Society for Pharmacoeconomics and Outcomes Research.17,18
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access









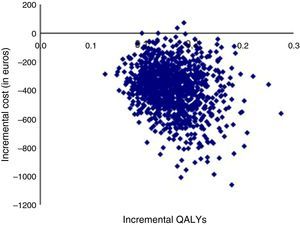 QALYs: quality-adjusted life years.' title='Probabilistic sensitivity analysis.
QALYs: quality-adjusted life years.' title='Probabilistic sensitivity analysis. 

