The benefits of implanted defibrillators in patients with ischemic heart disease (IHD) are well known. However, the evidence is less robust in patients with non-ischemic heart disease (non-IHD). We aimed to determine whether patients with non-IHD have a similar incidence of appropriate shocks and all-cause mortality compared to those with IHD.
MethodsIn a retrospective single-center study we analyzed all patients with implantable cardioverter-defibrillators or cardiac resynchronization therapy-defibrillators implanted for primary prevention between 2004 and 2014. The population was divided into two groups: patients with IHD and patients with non-IHD. The composite endpoint was appropriate shock and all-cause mortality.
ResultsTwo hundred and eighty-one patients were studied, of whom 187 (66%) had IHD. Patients with IHD were older, more frequently male and with more cardiovascular risk factors. Mean follow-up was 55±42 months. Thirty-four patients (18%) with IHD and 20 patients (21%) with non-IHD had an appropriate shock (p=0.64). Eighty-nine patients (47%) with IHD and 36 (38%) with non-IHD died during follow-up (p=0.19). The rate of shocks or death over time was similar in patients with IHD and non-IHD according to Kaplan-Meier survival curve analysis (log-rank p=0.10).
ConclusionIn this population, there were no differences in appropriate shocks or all-cause mortality in the two groups.
O benefício do desfibrilhador implantável está bem demonstrado nos doentes com cardiopatia isquémica. No entanto, a evidência deste benefício é menos robusta em doentes com cardiopatia não isquémica. Pretendeu-se determinar se os doentes com cardiopatia não isquémica apresentavam a mesma incidência de choque apropriado e mortalidade total, comparativamente àqueles com cardiopatia isquémica.
MétodosEstudo retrospetivo, em que foram analisados todos os doentes com cardioversor-desfibrilhador implantável ou terapia de ressincronização cardíaca com desfibrilhador, implantados para prevenção primária, entre 2004 a 2014, num único centro. A população foi dividida em dois grupos: doentes com cardiopatia isquémica e doentes com cardiopatia não isquémica. O endpoint primário combinado foi choque apropriado e morte por qualquer causa.
ResultadosForam estudados 281 doentes, dos quais 187 (66%) apresentavam cardiopatia isquémica. Os doentes com cardiopatia isquémica eram mais velhos, mais frequentemente do género masculino e com maior prevalência de fatores de risco cardiovasculares. O follow-up médio foi de 55±42 meses. Trinta e quatro doentes (18%) com cardiopatia isquémica e 20 doentes (21%) com cardiopatia não isquémica tiveram um choque apropriado (p=0,64). Oitenta e nove doentes (47%) com cardiopatia isquémica e 36 (38%) com cardiopatia não isquémica morreram durante o follow-up (p=0,19). A curva de Kaplan-Meier demonstra que a probabilidade de choque ou morte ao longo do tempo é semelhante nos dois grupos (logrank, p=0,14).
ConclusãoNa população estudada, não existiram diferenças na probabilidade de choque apropriado ou na mortalidade total nos doentes com cardiopatia não isquémica comparativamente aqueles com cardiopatia isquémica.
atrial fibrillation
cardiac resynchronization therapy
cardiac resynchronization therapy-defibrillator
body mass index
brain-type natriuretic peptide
implantable cardioverter-defibrillator
ischemic heart disease
heart failure
left ventricular ejection fraction
non-ischemic heart disease
New York Heart Association
obstructive sleep apnea
sudden cardiac death
transient ischemic attack
The 2016 European heart failure guidelines recommend the use of implantable cardioverter-defibrillators (ICDs) to reduce the risk of sudden cardiac death (SCD) and all-cause mortality in patients with symptomatic heart failure (HF) and left ventricular ejection fraction (LVEF) of ≤35% despite optimal medical therapy (class I recommendation).1 This is a level A recommendation for patients with ischemic heart disease (IHD), as the evidence of benefit is stronger (data derived from multiple randomized clinical trials or meta-analyses2–4), whereas for non-ischemic heart disease (non-IHD), it is a level B recommendation (data derived from a single randomized trial or large non-randomized studies3–5). The studies on which these recommendations are based were published over ten years ago, and the medical therapy and cardiac resynchronization therapy (CRT) in use at that time were inferior to present-day care, meaning that the benefit of an ICD was greater at that time. Furthermore, after the publication of the European guidelines, the DANISH trial demonstrated that prophylactic ICD implantation in patients with non-IHD did not significantly reduce overall mortality, although it reduced the risk of SCD.6 As a result, the benefit of ICDs in patients with non-IHD has recently been questioned.7
ObjectiveThe study aimed to determine whether the benefit of an ICD or cardiac resynchronization therapy-defibrillator (CRT-D) for primary prevention was different in patients with non-IHD compared to those with IHD in a real-world population.
MethodsStudy populationAll patients who received an ICD or CRT-D for primary prevention between 2004 and 2014 in a single center were studied.
Study designThis was a single-center retrospective observational study. The population was divided into two groups: patients with IHD and patients with non-IHD. Baseline characteristics (age, gender, body mass index [BMI], symptoms including New York Heart Association [NYHA] functional class, cardiovascular risk factors and comorbidities), echocardiographic assessment including LVEF, laboratory results including creatinine and brain-type natriuretic peptide (BNP) levels, current medication and type of device implanted (ICD or CRT-D) were collected for both groups at the time of device implantation.
DefinitionsIHD was defined as left ventricular systolic dysfunction (LVEF ≤35%) in the presence of ≥75% stenosis in the left main or proximal anterior descending artery or in two or more epicardial vessels, or as a history of myocardial infarction or previous coronary revascularization. Other cases were classified as non-IHD.
Hypertension was defined as resting systolic or diastolic blood pressure of ≥140/90 mmHg measured on at least two occasions or medication with antihypertensive drugs. Diabetes was defined as fasting blood glucose level of ≥126 mg/dl or medication with antidiabetic drugs. Patients were classified as current smokers or non-smokers. NYHA class was determined by the attending physician at the time of device implantation. Atrial fibrillation (AF), chronic obstructive pulmonary disease, obstructive sleep apnea, stroke, transient ischemic attack and peripheral arterial disease (PAD) were recorded as diagnosed by the attending physician. Transthoracic echocardiograms were reviewed and reassessed retrospectively by the study investigators and LVEF was calculated by the modified Simpson's biplane method.
Follow-upInformation on device-related events was collected by examining all follow-up records for patients with ICDs/CRT-Ds, as well as data from remote monitoring when available. Shocks were considered appropriate according to the decision of the investigator based on analysis of intracardiac electrograms and of the shock itself. All medical records pertaining to consultations, hospitalizations and emergency department visits were also analyzed, and records of deaths were verified.
OutcomesThe primary composite endpoint was appropriate shock and all-cause mortality, and the secondary endpoints were appropriate shock and all-cause mortality separately. Independent predictors of each of the secondary endpoints were assessed and causes of death were classified. The safety profile of device implantation was also analyzed, taking into account device-associated infection and inappropriate shocks. Subgroup analysis was performed of older patients, defined as those aged ≥68 years (the cutoff used in the DANISH trial6), and of patients with CRT-D as opposed to ICD.
Statistical analysisThe statistical analysis was carried out using IBM SPSS Statistics version 23 (IBM SPSS Inc., Chicago, IL). Continuous variables were expressed as mean ± standard deviation and categorical variables as number and percentage. Baseline characteristics were compared with the chi-square test for categorical variables and the Student's t test for continuous variables. Kaplan-Meier survival curves and the log-rank test were used to compare outcomes in the two study groups. Cox multivariate regression analysis was used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for appropriate shock and all-cause mortality. A p value <0.05 was considered statistically significant.
ResultsTwo hundred and eighty-one patients were studied, of whom 187 (66%) had IHD. The characteristics of the study population at the time of device implantation are shown in Table 1. Patients with IHD were older, more frequently male and with more cardiovascular risk factors and peripheral arterial disease, but there were no significant differences between the groups in terms of NYHA class, creatinine and BNP levels, LVEF and presence of AF. Patients with non-IHD more often received a CRT-D, while those with IHD more often received an ICD.
Characteristics of the study population at the time of device implantation.
| IHD (n=187) | Non-IHD (n=94) | p | |
|---|---|---|---|
| Age, years | 65±9 | 62±12 | 0.02 |
| Male gender, % | 167 (89) | 63 (67) | <0.0001 |
| BMI, kg/m2 | 26.9±4.1 | 27.7±4.3 | 0.14 |
| NHYA class | |||
| II | 101 (54) | 52 (55) | 0.97 |
| III | 81 (43) | 38 (40) | 0.72 |
| Laboratory data | |||
| BNP, pg/ml | 739±1651 | 758±1328 | 0.92 |
| Creatinine, mg/dl | 1.4±0.9 | 1.2±0.5 | 0.20 |
| Echocardiographic data | |||
| LVEF (%) | 27.3±6.9 | 26.9±6.7 | 0.61 |
| CV risk factors | |||
| Hypertension (%) | 140 (75) | 43 (46) | <0.0001 |
| Diabetes (%) | 97 (52) | 33 (35) | 0.01 |
| Dyslipidemia (%) | 142 (76) | 42 (45) | <0.0001 |
| Smoking (%) | 93 (50) | 15 (16) | <0.0001 |
| AF (%) | 85 (45) | 47 (50) | 0.55 |
| Comorbidities | |||
| COPD (%) | 22 (12) | 6 (6) | 0.17 |
| OSA (%) | 14 (8) | 12 (13) | 0.22 |
| Stroke/TIA (%) | 28 (15) | 5 (5) | 0.03 |
| PAD (%) | 14 (8) | 0 (0) | 0.01 |
| Type of defibrillator | |||
| ICD | 108 (58) | 32 (34) | 0.03 |
| CRT-D | 79 (42) | 62 (66) | 0.01 |
| Medication | |||
| ACEI/ARB (%) | 175 (94) | 91 (97) | 0.42 |
| Beta-blocker (%) | 158 (85) | 84 (89) | 0.40 |
| Spironolactone (%) | 71 (38) | 32 (34) | 0.59 |
| Furosemide (dose) | 37±29 | 37±25 | 0.97 |
| Antiarrhythmica (%) | 43 (23) | 26 (28) | 0.44 |
| OAC (%) | 95 (51) | 54 (57) | 0.36 |
Antiarrhythmics included amiodarone, digoxin and (for non-IHD) sotalol.
ACEI: angiotensin-converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; BMI: body mass index; BNP: brain-type natriuretic peptide; COPD: chronic obstructive pulmonary disease; CRT-D: cardiac resynchronization therapy-defibrillator; CV: cardiovascular; ICD: implantable cardioverter-defibrillator; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; OAC: oral anticoagulation; OSA: obstructive sleep apnea; PAD: peripheral arterial disease; TIA: transient ischemic attack.
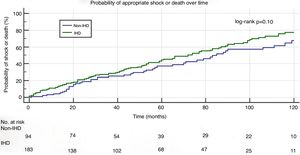
In a mean follow-up of 55±42 months, the primary composite endpoint of appropriate shock and all-cause mortality occurred in 150 patients (53%): in 105 (56%) in the IHD group and in 45 (46%) in the non-IHD group (p=0.15). Kaplan-Meier curve analysis (Figure 1) shows that the probability of appropriate shock or all-cause mortality was similar in the two study groups (log-rank p=0.10).
Secondary endpointsTwenty patients (21%) with non-IHD and 34 (18%) with IHD received appropriate shocks (p=0.64) during follow-up. The incidence of appropriate shock was 3.75 per person/year in the non-IHD group and 3.08 per person/year in the IHD group (p=0.19).
In patients who received appropriate shocks, the mean time to first shock was 3.5±2.7 years in the non-IHD group and 2.9±2.3 years in the IHD group (p=0.07). Kaplan-Meier curves for the occurrence of appropriate shock in both groups are shown in Figure 2A. The probability of appropriate shock was similar in the two groups (log-rank p=0.63).
Multivariate regression analysis showed that LVEF (HR 1.02, 95% CI 1.01-1.04, p=0.001 for each percentage point increase) and age (HR 0.97, 95% CI 0.95-0.99, p=0.04 for each year) were independently associated with appropriate shock.
Thirty-six patients (38%) with non-IHD and 89 (47%) with IHD died during follow-up (p=0.19). The mortality rate was 7.5 deaths per 100 person/years in the non-IHD group and 9.0 deaths per 100 person/years in the IHD group (p=0.11).
Mean time to death was 3.9±2.8 years in the non-IHD group and 4.1±3.1 years in the IHD group (p=0.67). The probability of all-cause death over time was similar in the two groups (log-rank p=0.09) (Figure 2B).
Factors independently associated with increased risk of death were elevated creatinine, age, NYHA class and higher furosemide dose at the time of device implantation. The use of beta-blockers was a protective factor against death (Table 2).
Of the 200 patients in whom the cause of death could be established (70% of deaths), 96 died of cardiovascular cause, 16 of SCD and 88 of non-cardiovascular causes. Causes of death were similar in the two study groups (Figure 3).
Subgroup analysisIn the subgroup of patients aged <68 years (n=181), there was a higher probability of appropriate shock (Figure 4C and D). However, there were no statistically significant differences between the IHD and non-IHD groups in either older (aged ≥68 years) or younger (aged <68 years) patients (Figure 4).
Subanalysis by type of device shows that patients receiving a CRT-D had a greater reduction in the combined endpoint of appropriate shock and all-cause mortality and in the secondary endpoint of all-cause mortality in the non-IHD group, the difference being clear from the beginning of follow-up (log-rank p=0.003 and p=0.002, respectively) (Figure 5A and E).
SafetyDevice-related infection occurred in six patients (1.6%), three in the IHD group and three in the non-IHD group. Of these six infections, four were in patients who received a CRT-D and two in patients who received an ICD (one single-chamber and one dual-chamber).
Inappropriate shocks occurred in 16 patients (9%) during follow-up. This was due to episodes of AF or atrial flutter in nine patients (56%) and to another supraventricular tachycardia in two, sinus tachycardia in one, lead fracture in two, T-wave oversensing in one and external interference in one.
DiscussionThere is still controversy concerning the benefits of defibrillators in patients with non-IHD. The clinical trials that showed benefit (SCD-HeFT4 and COMPANION8) also included patients with IHD and the reported effect was based on subgroup analysis of non-IHD subjects. Trials that showed no reduction in all-cause mortality (DEFINITE,5 CAT,9 AMIOVIRT10 and DANISH6) included non-IHD patients only. The fact that the DANISH trial was considerably more recent than the others means that other treatment for HF had improved, especially in terms of the number of patients treated with aldosterone antagonists and CRT, which obviously will have affected survival. The trial, published two years ago, showed no benefit of defibrillator implantation in reducing all-cause mortality (the trial's primary endpoint), although death from arrhythmia was reduced and all-cause mortality was in fact lower in the subgroup of patients aged <68 years (HR 0.64; 95% CI 0.45-0.90, p=0.01).
In an attempt to clarify this issue, a meta-analysis11 of the DANISH, CAT, DEFINITE and SCD-HeFT trials was recently published. According to the authors, primary prevention ICDs are efficacious at reducing all-cause mortality among patients with non-IHD, supporting the current guidelines.
Haugaa et al.12 studied the impact of the DANISH trial on clinical practice among European countries and concluded that the majority of centers did not implant ICDs for primary prevention on a regular basis in patients with non-IHD, despite current guidelines. By contrast, in patients with IHD, the guidelines for primary prevention ICD were followed, and no relevant change in indications were reported. It should be noted that most centers acknowledged having changed their indications for ICD implantation in non-IHD patients following the publication of the DANISH results. This is a good illustration of how a single trial can rapidly bring about widespread changes in clinical practice.
Our results suggest that implanted defibrillators have similar benefits in both IHD and non-IHD patients with regard to the incidence of appropriate shocks and all-cause mortality, which were similar in the two groups. Unlike in the DANISH trial, this benefit was also observed in older patients, in whom there were also no differences in the study endpoints.
The probability of appropriate shock was comparable in IHD and non-IHD patients, indicating that patients with non-IHD have a similar risk of fatal or potentially fatal arrhythmic events.
The incidence of appropriate shock in patients with non-IHD in our study was higher than in the DANISH trial6 (21% vs. 12%). This is most likely due to differences in the programming of detection zones for therapeutic shock, since this programming used to be more aggressive than that used nowadays. However, the programming was the same in our two study groups.
Our results also show that time to first appropriate shock is longer in patients with non-IHD (3.5±2.7 vs. 2.9±2.3 years in the IHD group), as seen in other studies,8 which highlights the need to control risk factors for non-sudden death and thus insure that the risk of death is not higher than that of appropriate shock.
There was also no difference between our study groups in all-cause mortality, except in the CRT-D subgroup, in which mortality was lower in those with non-IHD. These patients have been shown to have a better response to CRT-D than those with IHD, probably because of differences in the myocardial substrate.13 The lower mortality we observed in patients with non-IHD receiving a CRT-D, which was not seen in those in the same group who received an ICD, may thus be explained by their better response to CRT-D and hence better survival. This is also one of the explanations put forward for the low mortality in the DANISH trial, in which 60% of patients in both arms received a CRT-D.9,10
Regarding causes of death, a large proportion was of unknown cause, but SCD accounted for only 5% of deaths, which demonstrates the value of defibrillators in reducing arrhythmic SCD.
Despite its observational, retrospective and single-center nature, this study shines a light on the value of defibrillator use in real-world populations in recent years. As it was not ethically possible to compare groups with and without ICDs, since this would deprive some patients of a therapy that has been shown to be effective by numerous randomized trials and meta-analyses, we compared patients with IHD (in whom the benefits of an ICD have been clearly demonstrated) and those with non-IHD (concerning whom there is still debate). The study has some limitations, particularly in the programming of the ICD's detection zones (which in the early years of the study period tended to be more aggressive and hence led to shocks that could have been avoided), the fact that causes of death were not known for all patients, the lack of monitoring of medical therapy during follow-up, and the relatively small number of participants.
ConclusionsIn this population, there were no differences in appropriate shocks or all-cause mortality in patients with non-IHD compared to those with IHD. Implantation of a defibrillator (ICD or CRT-D) thus appears to be beneficial in both groups.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Marinheiro R, Parreira L, Amador P, Sá C, Duarte T, Fonseca M, et al. Será a implantação de desfibrilhador menos útil nos doentes com cardiopatia não isquémica? Rev Port Cardiol. 2018;37:835–841.








 IHD: ischemic heart disease.' title='Kaplan-Meier curve for the composite endpoint (appropriate shock and all-cause mortality).
IHD: ischemic heart disease.' title='Kaplan-Meier curve for the composite endpoint (appropriate shock and all-cause mortality).  IHD: ischemic heart disease.' title='Kaplan-Meier curves for the likelihood of appropriate shock (A) and all-cause mortality (B).
IHD: ischemic heart disease.' title='Kaplan-Meier curves for the likelihood of appropriate shock (A) and all-cause mortality (B). 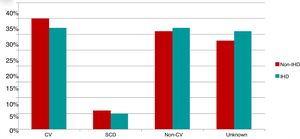 IHD: ischemic heart disease;
IHD: ischemic heart disease;  IHD: ischemic heart disease; Older patients: aged ≥68 years; Younger patients: aged 68 years.' title='Kaplan-Meier curves for the primary and secondary endpoints by age-group.
IHD: ischemic heart disease; Older patients: aged ≥68 years; Younger patients: aged 68 years.' title='Kaplan-Meier curves for the primary and secondary endpoints by age-group.  CRT-D: cardiac resynchronization therapy-defibrillator;
CRT-D: cardiac resynchronization therapy-defibrillator; 

