LDLr-/- mice are spontaneously hyperlipidemic and resistant to the development of neointimal lesions.
ObjectivesThis study aimed to determine the factor that prevents the inflammatory process and neointimal lesions and insulin resistance in LDLr-/- mice.
MethodsThree groups of 3-month-old male mice were used: wild-type mice (WT group); LDLr-/- mice fed a standard diet (S group); and LDLr-/- mice fed a high-fat diet (HF group). After 15 days, blood was collected for analysis of plasma lipids, glucose and insulin. The HOMA index was calculated to determine insulin resistance. The heart and aorta were removed for histological study. Histological sections of the heart were processed immunohistochemically with anti-CD40L antibodies to evaluate the inflammatory process. Histological sections of the aorta were stained with hematoxylin/eosin and picrosirius red to assess morphological and morphometric alterations.
ResultsThe S mice were resistant to the inflammatory process, as shown by low immunoreactivity to CD40L, with high plasma HDL levels, and did not develop insulin resistance, even with moderate hyperlipidemia compared to WT. The HF mice showed severe hyperlipidemia, increased cardiac immunoreactivity to CD40L, pronounced morphological changes in the aortic wall and insulin resistance, associated with a decrease in plasma HDL levels, compared to S. This severe hyperlipidemia in the HF mice can be considered the major metabolic factor inducing oxidative stress in the cardiovascular system, increasing the lipid peroxidation of HDL and hence its removal by the liver, with consequent lowering of plasma HDL levels.
ConclusionHigh HDL plasma levels are a protective factor against the development of cardiovascular inflammation and insulin resistance in LDLr-/- mice, preventing the development of neointimal lesions.
Camundongos knouckout para o gene do receptor de lipoproteína de baixa densidade (LDLr-/-) são hiperlipidêmicos espontâneos e resistentes ao desenvolvimento de lesões neointimais.
ObjetivosO presente estudo teve como objetivo determinar o fator que previne o processo inflamatório, as lesões neointimais cardiovasculares e a resistência insulínica nos camundongos LDLr-/-.
Material e métodosUtilizaram-se três grupos experimentais de camundongos machos com três meses de idade: Grupo WT, camundongos selvagens; Grupo S, camundongos LDLr-/- que receberam ração padrão; Grupo HL, camundongos LDLr-/- que receberam ração hiperlipídica. Após 15 dias, o sangue foi coletado para análise plasmática dos lipídeos, glicose e insulina. O índice de Homa foi calculado para determinar a resistência à insulina. O coração e aorta foram removidos e processados histologicamente. Cortes histológicos do coração foram processados imunoistoquimicamente com anticorpo anti-CD40L para avaliar a presença de processo inflamatório. Cortes histológicos das artérias foram corados com hematoxilina/eosina e picrosírius red para avaliar alterações morfológicas e morfométricas.
ResultadosOs camundongos S foram resistentes ao processo inflamatório, caracterizado por baixa imunorreatividade para o CD40L, com níveis plasmáticos de HDL elevados, e não desenvolveram resistência insulínica, mesmo com hiperlipidemia moderada em relação aos WT. Os camundongos HL apresentaram uma hiperlipidemia grave, aumento na imunorreatividade cardíaca para o CD40L, pronunciadas alterações morfológicas na parede da aorta e resistência insulínica, associadas a um decréscimo nos níveis plasmáticos do HDL em relação aos S. Esta hiperlipidemia grave dos camundongos HL pode ser considerada o fator metabólico indutor do maior estresse oxidativo no sistema cardiovascular, aumentando a peroxidação lipídica da molécula de HDL e consequentemente sua remoção hepática, com consequente diminuição dos níveis plasmáticos do HDL.
ConclusãoO nível plasmático elevado de HDL é o fator protetor contra o desenvolvimento de processos inflamatórios cardiovasculares e resistência insulínica nos camundongos LDLr-/-, impedindo o desenvolvimento das lesões neointimais.
There is a well-established relation between metabolic disturbances and cardiovascular disease, particularly atherosclerosis, hypertension, hyperlipidemia1 associated with low plasma levels of high-density lipoprotein (HDL), increased levels of low-density lipoprotein (LDL), endothelial dysfunction2 and diabetes3. The common denominators in all of these disorders are the inflammatory process and insulin resistance. The CD40 receptor and its ligand, CD40L, play an important role as inflammatory signals in different stages of cardiac hypertrophy4 and atherosclerosis5. CD40L is a transmembrane protein that has a pro-oxidant effect; the interaction with its receptor, CD40, induces an inflammatory response, promoting acute coronary syndromes6, activation of the NF-κB pathway7 and phosphorylation of IKK (inhibitor of κB kinase) and activating genes involved in inflammation and cardiac hypertrophy8. Studies have shown that inflammatory processes9 induce insulin resistance, which is associated with compensatory hyperinsulinemia. Hyperinsulinemia may continue to stimulate the mitogenic insulin-signaling pathway in an attempt to overcome to overcome inhibition of the metabolic insulin-signaling pathway, with deleterious effects on the cardiovascular system10.
The risk of developing cardiovascular disease is directly related to plasma LDL cholesterol levels and inversely related to HDL cholesterol levels11. Studies on LDL receptor knockout (LDLr−/−) mice have shown that they develop spontaneous moderate hyperlipidemia but are resistant to the development of carotid artery neointimal lesions12 and are subject to less arterial oxidative stress when fed a standard diet. However, when fed a high-fat diet, they become susceptible to neointimal lesions12 and present greater arterial oxidative stress, developing atherosclerotic plaques13.
This study aimed to determine the factor that prevents the inflammatory process and neointimal lesions and insulin resistance in LDLr−/− mice fed a standard diet.
MethodsAnimal protocolThe experiments were performed on wild-type mice (C57BL6 strain) and in LDLr–/– mice bred onto the C57BL6 background. The animals, 3-month-old males weighing 22±3 g, were obtained from the Jackson Laboratory (USA) and raised in the vivarium of the University of Alfenas (Alfenas, MG, Brazil), under controlled temperature conditions and with 12-hour cycles of light and darkness. The mice were divided into three groups: wild-type mice fed a standard diet (Nuvital®) (WT group, n=6); LDLr−/− mice fed a standard diet (Nuvital®) (S group, n=6); and LDLr−/− mice fed a high-fat diet with 20% total fat, 1.25% cholesterol and 0.5% cholic acid (HF group, n=6). All the animals received water ad libitum and their respective diet for 15 days.
After 15 days, the mice were anesthetized after 12 hours' fasting with intraperitoneal xilazine and ketamine (Bayer AS and Parke-Davis®, at concentrations of 6 and 40 mg/ kg, respectively). Blood was collected by puncture of the retro-orbital venous plexus for analysis of plasma glucose, insulin, triglycerides, total cholesterol and LDL, HDL and VLDL (very low-density lipoprotein) cholesterol. Thoracotomy was then performed and the heart and aorta were removed. The use of the animals and the experimental protocol were approved by the research ethics committee of the University of Alfenas.
Plasma analysisPlasma was obtained by centrifuging blood at 3000 rpm for 10 minutes. Plasma glucose was measured by enzymatic colorimetry, using the technique proposed by Trinder14. Plasma insulin was measured with a commercial ELISA kit (Dako Ltd, High Wycombe, Bucks, UK). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the formula: fasting insulinemia (mU/l) × fasting glycemia (mmol/l) / 22.5.
Enzyme assays were used to measure plasma lipids (triglycerides, total and HDL cholesterol), as described by Hedrick et al.15. LDL was determined using Friedewald's formula16 and VLDL using the method described by Tian et al.12.
Histological and immunohistochemical analysisImmediately after removal, the heart was dissected and the left ventricle and aorta were isolated and fixed for 24 hours in 10% formalin. They were then embedded in paraffin and cut into 4-μm sections for histological analysis, following the method described by Junqueira et al.17. The ventricular sections were treated with 3% hydrogen peroxide to block endogenous peroxidase activity and non-specific sites were blocked with 2% skim milk diluted with 0.1 M phosphate-buffered saline (PBS) at pH 7.4. The sections were incubated for 12 hours with rabbit polyclonal CD40L antibody (1:50 dilution; Santa Cruz Biotechnology) in a humidified chamber. After incubation with the primary antibody, they were then incubated with the biotinylated secondary antibody (Dako® LSAB+ kit) for one hour at 37 °C. To show immunoreactive areas, the sections were incubated with peroxidase-conjugated complex (Dako® LSAB+) for 45 minutes at 37 °C and placed in a chromogen solution (50 mg DAB in 50 ml PBS with 3 ml of 10% hydrogen peroxide) for three minutes. After staining with Harris hematoxylin (Sigma®) for 25 seconds, the sections were mounted and analyzed by optical microscopy using LGMC-image version 1.0 software to determine the proportion of the myocardium immunoreactive to CD40L18.
To assess morphological and morphometric alterations, the sections of the aorta were stained with hematoxylin/ eosin and the areas of the lumen, media and intima were measured using LGMC-image. The media is the area between the external elastic lamina and the internal elastic lamina, while the intima is the area between the internal face of the elastic lamina and the endothelial surface of the lumen12.
Sections of the aorta were also stained with picrosirius red to quantify collagen. Four photomicrographs were taken at the same pre-defined point in the cross-sections of the aorta of each animal, using a digital camera and Leica IM50 software (version 1.20). The stained sections were analyzed under polarized light using LGMC-image to determine the proportion of collagen18 in the media, intima and adventitia of the arterial wall.
All histological analyses were performed by a single operator using a double-blind method.
Statistical analysisThe data were expressed as means ± standard error of the mean (SEM). Analysis of variance followed by the Tukey test were used to compare means between the groups. Differences were considered significant for p<0.05.
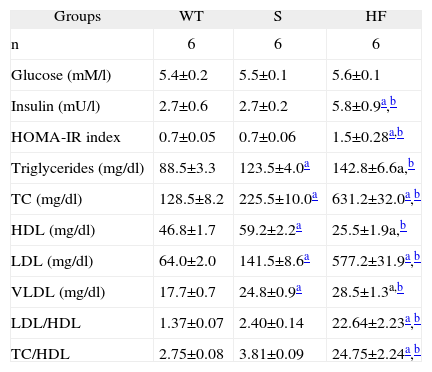
ResultsMice in the S group presented no differences from the WT group in plasma glucose or HOMA-IR, whereas the HF group presented markedly increased plasma insulin and HOMA-IR compared to both the other groups (Table 1).
Comparison of plasma glucose, insulin and lipids, HOMA-IR index, and cholesterol ratios (LDL/HDL e CT/HDL) in the experimental groups WT, S and HF
| Groups | WT | S | HF |
| n | 6 | 6 | 6 |
| Glucose (mM/l) | 5.4±0.2 | 5.5±0.1 | 5.6±0.1 |
| Insulin (mU/l) | 2.7±0.6 | 2.7±0.2 | 5.8±0.9a,b |
| HOMA-IR index | 0.7±0.05 | 0.7±0.06 | 1.5±0.28a,b |
| Triglycerides (mg/dl) | 88.5±3.3 | 123.5±4.0a | 142.8±6.6a,b |
| TC (mg/dl) | 128.5±8.2 | 225.5±10.0a | 631.2±32.0a,b |
| HDL (mg/dl) | 46.8±1.7 | 59.2±2.2a | 25.5±1.9a,b |
| LDL (mg/dl) | 64.0±2.0 | 141.5±8.6a | 577.2±31.9a,b |
| VLDL (mg/dl) | 17.7±0.7 | 24.8±0.9a | 28.5±1.3a,b |
| LDL/HDL | 1.37±0.07 | 2.40±0.14 | 22.64±2.23a,b |
| TC/HDL | 2.75±0.08 | 3.81±0.09 | 24.75±2.24a,b |
Data are means±SEM.
HDL: high-density lipoprotein; HOMA-IR: homeostasis model assessment of insulin resistance; LDL: low-density lipoprotein; TC: total cholesterol; VLDL: very low density lipoprotein.
Analysis of the mice's lipid profile revealed moderate hyperlipidemia in the S group, with higher plasma triglycerides and total cholesterol (TC) than the WT group. The HF group showed severe hyperlipidemia compared to the S and WT mice. In terms of cholesterol fractions, higher HDL, LDL and VLDL were seen in the S group than in the WT group, and the HF group had lower plasma HDL and markedly higher LDL and VLDL concentrations than both the other groups. The LDL/HDL and TC/HDL ratios were higher in the HF group than in the S group but there was no difference between groups S and WT (Table 1).
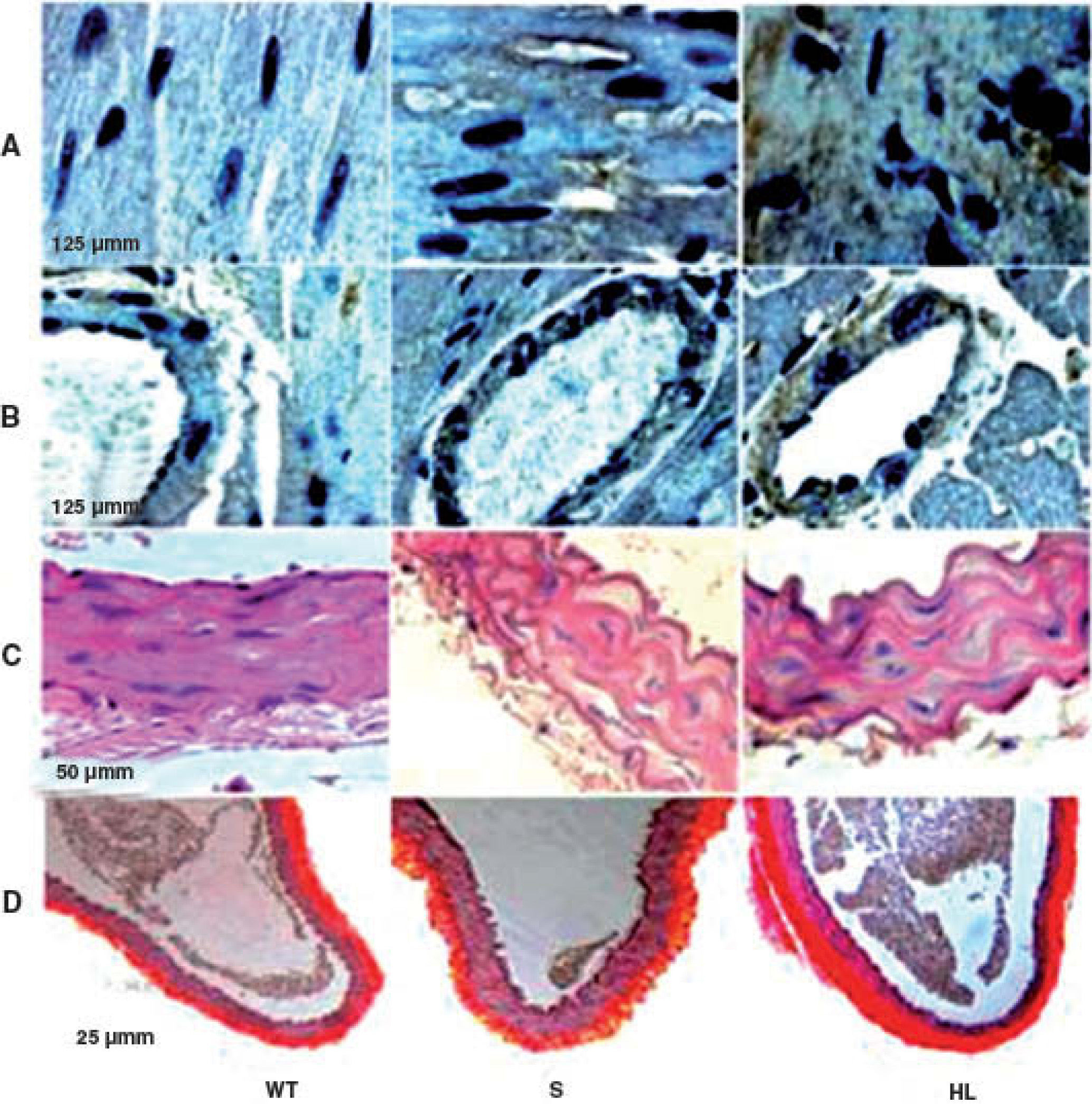
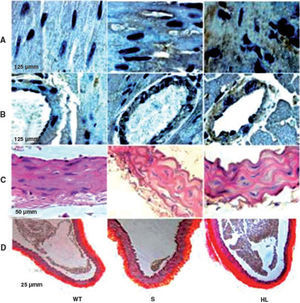
Group S showed higher immunoreactivity to CD40L in the myocardium and coronary artery than group WT. This immunoreactivity was even more marked in the HF group (brown in Figures A and B) (Table 2).
Photomicrographs of cross-sections of the left ventricle, showing the areas immunoreactive to CD40L in the myocardium and coronary artery (brown) (A and B); photomicrographs of sections of the aorta stained with hematoxylin/eosin (C) and picrosirius red (D). HL: hyperlipidemic; S: standard; WT: wild type.
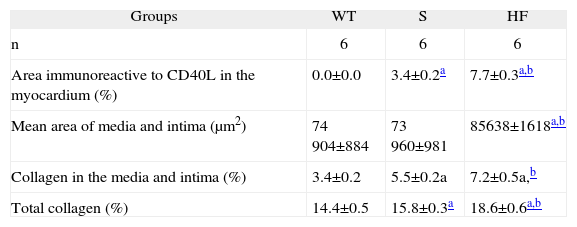
Comparison of areas immunoreactive to CD40L in the myocardium, areas of intima and media, percentage of collagen in the media and intima, and total collagen in the aorta, in the experimental groups WT, S and HF
| Groups | WT | S | HF |
| n | 6 | 6 | 6 |
| Area immunoreactive to CD40L in the myocardium (%) | 0.0±0.0 | 3.4±0.2a | 7.7±0.3a,b |
| Mean area of media and intima (μm2) | 74 904±884 | 73 960±981 | 85638±1618a,b |
| Collagen in the media and intima (%) | 3.4±0.2 | 5.5±0.2a | 7.2±0.5a,b |
| Total collagen (%) | 14.4±0.5 | 15.8±0.3a | 18.6±0.6a,b |
Data are means±SEM.
Morphological and morphometric analysis of the aortic intima and media shows that, unlike the WT group, the S and HF groups presented arterial remodeling characterized by increased area of the intima and between the elastic fibers of the media (Figure C and Table 2), and by greater collagen deposition in the media and intima and more total collagen in the intima, media (Table 2) and adventitia (Table 2 and Figure D). This remodeling was much more marked in the HF group than in the S group (Table 2, Figure C and D).
DiscussionIn the present study, mice in the S group were resistant to the development of lesions in the aortic wall and to the inflammatory process, as shown by low immunoreactivity of cardiac tissue to CD40L. Interestingly, the S group had high plasma HDL levels, and did not develop insulin resistance, even with moderate hyperlipidemia compared to WT. The HF mice showed severe hyperlipidemia, increased cardiac immunoreactivity to CD40L, pronounced morphological changes in the aortic wall and insulin resistance, associated with a marked decrease in plasma HDL levels, compared to S.
Studies have shown that atherosclerosis is an inflammatory disease2. The main theories for atherogenesis that have been proposed in recent years are reverse cholesterol transport19 and LDL oxidation2,20. In both mechanisms oxidized LDL and HDL play a central role, as initiating and delaying atherogenesis respectively. The raised HDL levels in the S group mice may have had a cardiovascular protective effect due to its antioxidant action20. This is related to the protein apoA-I, which eliminates lipid hydroperoxides from LDL21. The HDL molecule also metabolizes lipid hydroperoxides that oxidize LDL phospholipids through the action of the enzymes paraoxonase-122 and paraoxonase-323. Our results corroborate those of Krieger et al.13, who demonstrated that LDLr−/− mice fed a standard diet showed less oxidative stress in the aorta compared to those fed a high-fat diet.
The anti-inflammatory effect of HDL in our study may be related to its role in reducing oxidative stress24. It also inhibits the expression of adhesion molecules (VCAM-1, ICAM-1 and E-selectin) on the surface of endothelial cells induced by proinflammatory cytokines25, thereby reducing the transmigration of monocytes to the subendothelial region. This may explain the prevention of cardiovascular inflammation, as shown by the low expression of CD40L in the coronary artery and myocardium, and the absence of morphological changes in the aortic wall despite moderate hyperlipidemia, seen in the S group mice. The determining factor in the lower arterial oxidative stress described by Krieger et al.13 and the greater resistance to the development of arterial lesions observed by Tian et al.12, in LDLr−/− mice fed a standard diet, may thus be their increased plasma HDL levels, as seen in our study, compared to LDLr-/- mice fed a high-fat diet.
Insulin induces tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1), while factors that lead to insulin resistance, including TNF-α, free fatty acids, oxidative stress and inflammation, activate serine/threonine kinases that phosphorylate IRS-1, inhibiting its function26. Insulin resistance associated with hyperinsulinemia was not observed in the S group in our study, indicating that the antioxidant and anti-inflammatory effects of HDL in these animals may have prevented oxidation of insulin receptor substrates. Several studies in vitro and in animal models have demonstrated that various proinflammatory cytokines27 are involved in the pathogenesis of insulin resistance, and a range of anti-inflammatory therapies have been shown to improve insulin sensitivity28.
Studies indicate that LDLr−/− mice fed a high-fat diet develop extensive atherosclerotic aortic lesions13, left ventricular hypertrophy4, neointimal lesions and coronary artery remodeling12 associated with increased oxidative stress. In the present study, mice in the HF group were more susceptible to aortic wall lesions, as described by Tian et al.12. They also presented cardiac inflammation, hyperinsulinemia and insulin resistance. These morphological and metabolic alterations were associated with a marked increase in plasma LDL and a dramatic fall in HDL. This induced oxidative stress in the myocardium4 and arteries13, due to mechanisms that increased production of reactive oxygen species or weakened endogenous antioxidant factors.
The increased expression of CD40L in the cardiovascular system of mice in the HF group had a pro-oxidant as well as a proinflammatory effect29. This indicates that the severe hyperlipidemia in the HF mice can be considered the major metabolic factor inducing oxidative stress in the cardiovascular system, increasing the lipid peroxidation of HDL and hence its removal by the liver30, with consequent lowering of plasma HDL levels and weakening of its antioxidant and anti-inflammatory protective effect, promoting cardiovascular inflammation and insulin resistance.
ConclusionHigh HDL plasma levels were shown to be a protective factor against the development of cardiovascular inflammation and insulin resistance in LDLr−/− mice, preventing the development of neointimal lesions. Close monitoring of plasma HDL levels is therefore essential in dyslipidemic patients, since excessive dietary fat could reduce HDL levels and thus trigger the inflammatory process and the development of insulin resistance and hyperinsulinemia.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful to the Fundacao de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) and Universidade José do Rosário Vellano for their financial support for this project; to Prof. Vinícius Vieira Vignoli for his linguistic revision; and to Antonio Marcus Martins for his technical assistance in the histological preparations.