We describe our center's initial experience with alcohol septal ablation (ASA) for the treatment of obstructive hypertrophic cardiomyopathy. The procedure, its indications, results and clinical outcomes will be addressed, as will its current position compared to surgical myectomy.
ObjectiveTo assess the results of ASA in all patients treated in the first four years of activity at our center.
MethodsWe retrospectively studied all consecutive and unselected patients treated by ASA between January 2009 and February 2013.
ResultsIn the first four years of experience 40 patients were treated in our center. In three patients (7.5%) the intervention was repeated. Procedural success was 84%. Minor complications occurred in 7.5%. Two patients received a permanent pacemaker for atrioventricular block (6% of those without previous pacemaker). The major complication rate was 5%. There were no in‐hospital deaths; during clinical follow‐up (22±14 months) cardiovascular mortality was 2.5% and overall mortality was 5%.
Discussion and ConclusionThe results presented reflect the initial experience of our center with ASA. The success rate was high and in line with published results, but with room to improve with better patient selection. ASA was shown to be safe, with a low complication rate and no procedure‐related mortality. Our experience confirms ASA as a percutaneous alternative to myectomy for the treatment of symptomatic patients with obstructive hypertrophic cardiomyopathy refractory to medical treatment.
A ablação septal alcoólica (ASA) é a forma percutânea de tratamento invasivo da cardiomiopatia hipertrófica obstrutiva (CMHO). A propósito da descrição da experiência no nosso centro, procurar‐se‐á rever as indicações, os aspetos técnicos e práticos e os resultados da ASA, assim como a sua posição atual em comparação com a miectomia cirúrgica.
ObjetivoAvaliar os resultados da ASA numa série de doentes consecutivos tratados nos primeiros quatro anos de atividade.
MétodosEstudo retrospetivo de todos os doentes, consecutivos e não selecionados, com CMHO, tratados por ASA, entre janeiro de 2009 e fevereiro de 2013.
ResultadosDurante o período de quatro anos foram tratados com ASA 40 doentes. Em três doentes (7,5%) repetiu‐se o procedimento. A taxa de sucesso foi de 84%. A taxa de complicações minor foi de 7,5%. Foi necessário implantar pacemaker definitivo por bloqueio‐auriculoventricular em dois doentes (6%, do subgrupo sem pacemaker prévio). A taxa de complicações major foi de 5%. Não houve mortalidade intra‐hospitalar nesta população. Durante o seguimento clínico (22 ± 14 meses) a mortalidade cardiovascular foi de 2,5%. A mortalidade total foi de 5%.
Discussão e conclusãoOs resultados apresentados refletem a experiência inicial do tratamento com ASA no nosso centro. O procedimento foi bem‐sucedido na maioria dos doentes, sendo a taxa de sucesso semelhante à descrita em outras séries, mas ainda com possibilidade de beneficiar de uma melhor seleção dos doentes. A intervenção também se revelou segura, com uma baixa ocorrência de complicações e sem mortalidade associada. A ASA é uma alternativa percutânea no tratamento invasivo dos doentes com CMHO refratária à terapêutica médica.
Hypertrophic cardiomyopathy (HCM) was first described over 50 years ago,1 and it was soon clear that one of its most characteristic features was the presence of dynamic subaortic obstruction in a significant number of patients.2
The genetic and phenotypic heterogeneity of this primary cardiomyopathy complicates clinical assessment and treatment, and has given rise to considerable debate.3 One of the more recent controversies concerns invasive treatment of obstructive hypertrophic cardiomyopathy (OHCM); the introduction of percutaneous alcohol septal ablation (ASA) as an alternative to the established surgical technique of septal myectomy led to much discussion concerning the relative merits of the two treatments that continues to this day.
Most patients with HCM present significant intraventricular obstruction at rest or with provocation, usually the Valsalva maneuver (or, strictly speaking, the effort required for the maneuver).4 This obstruction is caused by the systolic anterior motion (SAM) of the anterior leaflet of the mitral valve, which results from the force of left ventricular (LV) ejection and the narrowing of the LV outflow tract (LVOT); elongation of the mitral valve leaflets may also contribute in some cases.5 The obstruction increases intracardiac pressure, oxygen consumption and cardiac work, and is also often associated with mitral regurgitation, coronary flow abnormalities and diastolic dysfunction. The degree of obstruction correlates with symptom severity and worse survival.6
Identification of patients with OHCM is important, because the obstruction is itself a therapeutic target; reducing it frequently results in improvement or complete resolution of symptoms. Medical therapy is effective in most cases and should therefore be the first‐line treatment, using inotropic depressors such as beta‐blockers, non‐dihydropyridine calcium channel blockers or disopyramide (the latter unavailable in Portugal).
However, in 5–10% of patients medical therapy is ineffective, not tolerated or contraindicated.7 In such cases, an invasive approach is an alternative for symptomatic patients with impaired quality of life. The aim is to reduce the thickness of the basal portion of the interventricular septum and hence the obstruction. The first technique used was surgical myectomy, in which a part of the septal muscle is excised; this was the only option for decades. In experienced centers, mortality is ≤2% in young patients without significant comorbidities.8 It is not considered an easy procedure, since the surgeon faces considerable variation in the morphology of the LVOT with limited transaortic access. However, in centers with decades of experience that have treated hundreds of cases the procedure is highly effective, significantly reducing gradients and symptoms in 90–95% of patients.7
The need for another therapeutic option for patients with contraindication or high risk for surgery prompted the development of a percutaneous alternative. ASA consists of injection of alcohol into a coronary artery in order to cause limited myocardial necrosis in the basal septum, which when healed reduces septal thickness and hence the subaortic gradient. The technique has been the subject of controversy since its introduction, but its results are such that it has become a viable alternative to surgical treatment of patients with OHCM.
ObjectiveThe aim of this study is to assess the results of ASA in all patients treated in the first four years of activity at a single cardiological reference center.
MethodsWe performed a retrospective study based on data collected prospectively of all consecutive and unselected patients with OHCM and indication for an invasive approach, treated by ASA between the beginning of activity in January 2009 and February 2013.
Demographic variables, clinical indications, minor and major complications, procedural success and long‐term outcome were assessed. Success was defined as symptomatic improvement (reduction of at least one New York Heart Association [NYHA] or Canadian Cardiac Society [CCS] class) together with a reduction of over 50% in subaortic gradients at rest or with provocation.3 Major complications were considered to be those that resulted in death or significant morbidity or were life‐threatening during the procedure or follow‐up.
Categorical variables are presented as counts and percentages, and continuous variables as means ± standard deviation. The statistical analysis was performed using SPSS version 20.
ResultsDuring the four‐year study period, 43 patients with indication for invasive treatment were assessed, three of whom were excluded because a target vessel was lacking or perfused remote myocardial segments. A total of 43 procedures were performed in 40 patients. The main characteristics of the study population are presented in Table 1.
Baseline characteristics of the study population (n=40).
| Age (years) | 61.5±12.7 |
| Age ≥75 years | 6 (15) |
| Female | 27 (70) |
| NYHA class III/IV | 39 (97.5) |
| CCS class II/III | 10 (25) |
| Syncope | 1 (2.5) |
| Previous history | |
| Hypertension | 26 (65) |
| Diabetes | 3 (7.5) |
| Coronary disease | 4 (10) |
| Atrial fibrillation | 7 (17.5) |
| LBBB | 2 (5) |
| Previous permanent pacemaker | 3 (7.5) |
| Previous ICD | 1 (2.5) |
| Echocardiogram | |
| Maximum septal thickness | 21.6±2.66 |
| Gradient at rest | 89.6±33.6 |
| Gradient with provocation | 109±30 |
| Mitral regurgitation grade ≥2 | 1 (2.5) |
| Ejection fraction <50% | 0 (0) |
| Therapy | |
| Beta‐blocker | 29 (72.5) |
| Calcium channel blocker | 27 (67.5) |
| Dual therapy | 17 (43) |
| DDD pacemaker | 2 (5) |
| Myectomy | 0 (0) |
Values are counts (%) or means ± standard deviation. CCS: Canadian Cardiac Society; LLLB: left bundle branch block; ICD: implantable cardioverter‐defibrillator; NYHA: New York Heart Association.
The most frequent indication was exertional dyspnea; one patient (2.5%) was in NYHA class IV and in another (2.5%) the sole indication was exertional angina. Six patients (15%) had a previously implanted pacemaker, one with a DDD pacemaker implanted prior to the intervention due to existing left bundle branch block, which put her at high risk for complete atrioventricular block (AVB) following the procedure.
Mean subaortic gradient at rest was 90±34 mmHg and mean septal thickness was 21.6±4 mm. Three patients (7.5%) had a resting gradient of <50 mmHg and in these cases the indication was due to the gradient obtained with provocation. Ten patients (30%) were considered not ideal for myectomy due to age, obesity or comorbidities, and two (5%) were formally rejected for surgery.
Data on the intervention and in‐hospital course are presented in Table 2. Alcohol was injected into a single target vessel, which was changed after intramyocardial contrast injection in four cases (10%). Two patients (6% of those without previous pacemaker) received a permanent pacemaker for AVB, one late, on the seventh day after the procedure and after reintroduction of beta‐blocker therapy. In four patients (10%) with AVB after 24 hours the initial approach was conservative based on their risk score, waiting for resolution of the edema associated with the necrotic area and re‐establishment of atrioventricular conduction, which in some cases did not occur until the fifth day.
Intervention and hospitalization (n=40).
| No. of septal arteries | 43 |
| Volume of alcohol injected (ml) | 2.1±0.3 |
| Peak CK (U/ml) | 1130±438 |
| AVB risk score | |
| Low | 27 (75a) |
| Intermediate | 7 (19a) |
| High | 2 (6a) |
| Permanent pacemaker | 2 (6a) |
| Sustained ventricular tachycardia | 0 (0) |
| Ventricular fibrillation | 1 (2.5) |
| Complications during hospitalization | |
| Major | 2 (2.5) |
| Minor | 3 (7.5) |
| In‐hospital mortality | 0 (0) |
Values are counts (%) or means ± standard deviation. AVB: atrioventricular block; CK: creatinine kinase.
The rate of minor complications was 7.5%. In two patients infections were resolved by antibiotic therapy, and in one patient rapid atrial fibrillation was controlled with pharmacological therapy. The major complication rate was 5%, including one patient with tamponade caused by the introduction of the catheter and resolved by pericardiocentesis and pericardial drainage, and one with an inferior infarction caused by recruitment of the collaterals of the target vessel to the posterior interventricular branch, leading to an episode of ventricular fibrillation a few hours after the intervention, resolved by shock therapy. All patients underwent Holter ECG monitoring before discharge; no complex ventricular arrhythmias were documented. There were no in‐hospital deaths.
Table 3 presents the data on clinical follow‐up. Most patients experienced symptomatic improvement associated with a significant reduction in subaortic gradient. Procedural success was 84%. Information on post‐procedural gradient is unavailable for four patients, three because the procedure was performed less than three months ago and one because of difficulty in repeating echocardiography due to severe neurological sequelae of a stroke. Two patients (5%) suffered stroke, both more than 30 days after the intervention; four (10%) were readmitted due to paroxysmal atrial fibrillation, one of whom underwent successful ablation. One patient (2.5%) was rehospitalized for implantation of an implantable cardioverter‐defibrillator due to fainting and non‐sustained ventricular tachycardia, both documented before ASA. Overall mortality was 5%: one patient died from probable pulmonary embolism 13 months after the intervention and one died of colon cancer.
Clinical course (n=39).
| Mean follow‐up (months) | 22±14 |
| Symptomatic improvement | |
| Dyspnea | 31 (86a) |
| Angina | 9 (90a) |
| Syncope | 1 (100a) |
| Reduction in gradient | 33 (89) |
| Successful ASA | 31 (84) |
| Permanent pacemaker | 0 (0) |
| ICD | 1 (2.5) |
| Repeat ASA | 3 (7.5) |
| Myectomy | 1 (2.5) |
| No cardiovascular events | 31 (78) |
| Rehospitalization for cardiac cause | 5 (12.5) |
| Mortality | |
| Cardiovascular | 1 (2.5) |
| Sudden death | 0 (0) |
| Total | 2 (5) |
Values are counts (%) or means ± standard deviation. ASA: alcohol septal ablation; AVB: atrioventricular block; ICD: implantable cardioverter‐defibrillator.
Since the first procedure performed by Ulrich Sigwart in 1994 and described, with two other cases, in 1995,9 ASA has been refined and is now more effective and safe, to the point that its results are now comparable to surgical myectomy. It is no longer seen as a pioneering and poorly‐defined technique, but has become an established procedure over the course of nearly two decades, during which it has been used to treat more patients than surgery.10
Patient selectionThe indications for ASA are the same as for surgical myectomy, although it is important to stress that patients with no or mild symptoms are not indicated for any invasive treatment.7 The presence of obstruction is an independent prognostic marker of progression to heart failure and mortality,6 but there is currently insufficient evidence that intervention alters prognosis in these patients; its role is to treat symptoms that significantly affect patients’ quality of life.
Selected patients should have exertional dyspnea (NYHA class III or IV), exertional angina (CCS class II or III) or exertional syncope for which an arrhythmic cause has been excluded. There should be dynamic obstruction of ≥50 mmHg at rest and/or ≥70 mmHg with provocation, although the current trend is to consider patients with lower values – ≥30 mmHg at rest and/or ≥50 mmHg with provocation. In our study population, which reflects our initial experience and hence a learning curve, we opted for a more conservative selection process using the higher values. It should be stressed again that invasive treatment is only indicated when medical therapy is ineffective, not tolerated or contraindicated. In reference centers, optimization of medical therapy in patients referred for invasive treatment can improve clinical status in a considerable proportion and remove the initial indication for ASA or myectomy.11
Given the risk of causing an iatrogenic interventricular communication, basal septal thickness should be ≥16 mm for both ASA and myectomy. Mitral regurgitation, even if significant, is not a contraindication for ASA so long as it is functional and due to SAM, since the latter will improve with reduction of the obstruction. However, primary mitral regurgitation, or another indication for cardiac surgery, is reason to perform myectomy. In patients with severe hypertrophy (>30 mm) or in whom the papillary muscle is implanted directly into the anterior mitral leaflet causing LVOT obstruction, ASA is less likely to succeed and surgery is preferable. For ASA to be feasible it is also essential that the obstruction should be in the region of the basal septum and that there should be one or more septal arteries that can be selectively catheterized and that irrigate only that part of the septum. ASA can be performed after unsuccessful myectomy.12
Although cases of ASA in children have been reported, many operators, including the authors, consider that the low surgical risk at pediatric ages and the lack of knowledge of the long‐term consequences of myocardial necrosis in a young and still developing heart mean that surgery is the preferred option.
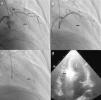
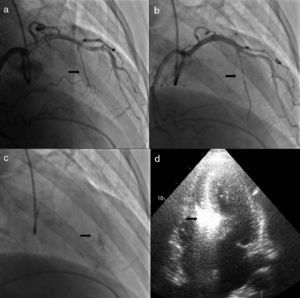
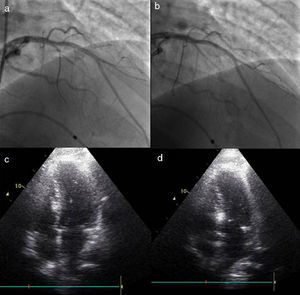
ProcedureThe procedure begins with coronary angiography in order to identify the target vessel, usually the first septal branch to the basal septum, in which systolic milking is visible. The target septal branch may arise from a diagonal, the circumflex, or even the right coronary artery, although the latter was not observed in our population. Following assessment of the coronary anatomy, including ease of access to and caliber of the target vessel, the material to be used in the procedure can be selected. In our center we generally use 6F Judkins and EBU guiding catheters, BMW® or Pilot 50® guidewires (Abbott Vascular, Santa Clara, CA, USA) and Apex Flex® over‐the‐wire balloon catheters (Boston Scientific, Natick, MA, USA). A femoral approach is preferred for greater stability during coronary catheterization. When significant coronary lesions are detected with indication for revascularization, we consider that this should be performed first and ASA postponed pending reassessment of symptoms. In one of our patients the origin of the target septal branch was close to a lesion in the anterior descending artery and stenting would have compromised subsequent access, and so it was decided to perform ASA first and then to treat the lesion in the same procedure (Figures 1 and 2).
Procedure: (a) angiography, right oblique cranial view of the left coronary artery, showing the target vessel; (b) selective catheterization of the target vessel and placement of balloon catheter; (c) selective angiography of the target vessel, showing injection of contrast through the lumen of the balloon catheter; (d) transthoracic echocardiography, 4‐chamber view, showing hyperechogenic basal septum after injection of contrast.
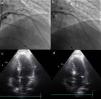
Change in the target vessel after contrast injection: (a) selective catheterization of the second septal branch; (b) selective catheterization of the first septal branch; (c) opacification of the right side of the septum after injection of contrast in the second septal branch; (d) opacification of the appropriate region of the septum (below the anterior leaflet of the mitral valve), following contrast injection in the first septal branch.
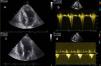
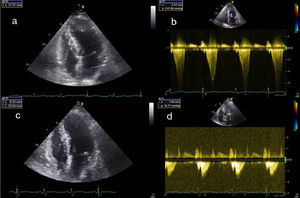
All patients should undergo prior Doppler echocardiography to confirm the diagnosis and to accurately locate and quantify the obstruction(Figure 3). When doubts remain concerning the latter, hemodynamic assessment is performed to record aortic and intraventricular pressures simultaneously and to quantify the gradient at rest, with the Valsalva maneuver, and after a ventricular premature beat.
Echocardiographic images: (a) transthoracic echocardiography, 4‐chamber view, before the intervention; (b) continuous Doppler study showing subaortic gradient before the intervention; (c) transthoracic echocardiography, 4‐chamber view, three months after the intervention, showing decreased thickness of the basal septum; (d) continuous Doppler study three months after the intervention, showing reduction in the subaortic gradient.
During the procedure, transthoracic echocardiographic study is first performed to assess the echogenicity of the cardiac structures for subsequent comparison and to exclude pericardial effusion. After the target vessel is identified a temporary pacing catheter is placed in the right ventricle via a transvenous femoral or jugular approach. Following confirmation that this is stable and effective, heparin 70–100 U/kg is administered, then the target septal artery is approached with a guidewire previously inserted into the balloon catheter. The latter is then introduced over the guidewire into the target vessel and positioned at the desired location, which is selected according to the diameter of the vessel and the existence of bifurcations that may or may not be subject to alcohol injection, depending on their size and course. The diameter of the balloon should be 0.5 mm greater than that of the target vessel at the point where inflation will take place; a smaller diameter may not completely occlude the vessel, allowing alcohol to flow back to the anterior descending artery, while an oversized balloon may be displaced during alcohol injection.
Following balloon inflation at low pressure (4–6 atm), the guidewire is withdrawn and angiographic contrast is injected in order to visualize the distal circulation of the target vessel, exclude the existence of collaterals and confirm total occlusion of the vessel with no backflow of contrast to the anterior descending artery. Then 1–2 cc of myocardial contrast is injected; initially Levovist® (Bayer AG, Schering AG, Germany) was used, and after this was discontinued, SonoVue® (Bracco, Milan, Italy). Transthoracic echocardiography is used to assess the hyperechogenicity of the target region, the basal septum where SAM occurs, and flow turbulence, and to exclude the involvement of remote regions such as the LV free wall, the right ventricle and the papillary muscles.
Once the decision is made to go ahead with alcohol ablation, analgesia is administered with morphine (4–6 cc intravenously, which is well tolerated in most patients) and the correct functioning of the temporary pacemaker is checked. This step is extremely important, since transient AVB is common. Slow injection (1 cc/min) of 1 cc alcohol per cm thickness of the target septal tissue follows, constantly monitoring that the balloon is correctly positioned and well inflated. Following alcohol administration and after a 10‐min wait, the guidewire is reinserted into the lumen of the balloon catheter, which is deflated and the whole system is removed. A final angiogram is performed to exclude coronary dissection or impaired coronary flow.
Patients remain in the coronary care unit for the first 24–48 hours, under continuous ECG surveillance. The creatinine kinase curve is monitored at four‐hour intervals until its peak, the time of which is one of the parameters in the AVB risk score used in our population.13 In low‐risk patients the pacing catheter is removed 24–48 hours after the intervention and the patient returns to the ward under telemetric ECG monitoring and restarts his or her previous medication (beta‐blockers and/or calcium channel blockers), which are usually suspended before the procedure. If there are no complications, the patient is discharged on the sixth or seventh day.
The procedure described above undoubtedly differs in small details from that used by other centers and operators, but the basic steps will be common to all for whom safety is a priority. We highlight three changes that have helped improve safety and outcomes compared to the first published series. Firstly, we use a smaller quantity of alcohol, reducing the area of necrosis and hence the risk of affecting atrioventricular conduction tissue. Similarly, the immediate objective of reducing the subaortic gradient, with repeated alcohol injections if necessary, has been abandoned in favor of assessing the result after septal healing and remodeling. The second change, introduced by Faber et al. in 1998, was the use of intraprocedural myocardial contrast injection and transthoracic echocardiography to determine the target vessel and prevent necrosis in remote myocardial regions.14 This is now an important step in the procedure; in our series the target vessel was changed in 10% of cases, and the intervention was abandoned in three patients (not included in the study) because echocardiographic assessment failed to identify a suitable vessel. Thirdly, the introduction of risk scores for high‐degree AVB has made it possible to distinguish patients in whom it is better to await resolution of the acute phase of septal edema, after which conduction disturbances resolve in the majority of cases, from those in whom a permanent pacemaker should be implanted immediately.13
EfficacyImmediate gradient reduction is no longer considered essential for the efficacy of the procedure. Following ASA, the gradient goes through three stages: immediately after alcohol administration it falls due to myocardial stunning; it then rebounds over 5–10 days due to edema in the necrosed area; and finally scarring and remodeling occur after around three months, usually leading to improvement in the gradient up to a year after the intervention.15
In 2006, Alam et al. published a meta‐analysis of 42 studies on outcomes after ASA in 2959 patients.16 At 12 months, there was a sustained decrease in resting and provoked LVOT gradient (65.3 to 15.8 mmHg and 125.4 to 31.5 mmHg, respectively) accompanied by reduction in basal septal thickness (20.9 to 13.9 mm), improvement in NYHA class (2.9 to 1.2), and increase in exercise capacity (325.3 to 437.5 seconds). ASA is associated with sustained improvements in symptoms and gradient in >85% of patients.17,18 Repeat ASA due to persistence or recurrence of symptoms is necessary in around 7% of cases, a similar figure to myectomy.16
ASA results in increased LVOT area and reduced gradient19,20; besides symptomatic improvement, its benefits include increased coronary flow reserve21 and LV size and reduced LV mass,22 end‐diastolic pressure,14 left atrial size14 and mitral regurgitation.23 The clinical effects observed are also due to improved LV diastolic function, which is comparable to that obtained by myectomy.24,25
If the procedure is unsuccessful, myectomy remains an option, albeit with a greater risk of need for permanent pacing, due to the combination of the complete right bundle branch block that often results from alcohol injection and the left bundle branch block caused by the surgical incision in the left side of the septum.
Safety and complicationsThe most common complication in ASA is irreversible high‐degree AVB, but the improvements in the technique described above have reduced the frequency of this complication to <10%, far lower than the initial results of some centers.17,18 In some cases persistence of AVB beyond the first 24 hours is due to the effect of perinecrotic inflammation on adjacent conduction tissue, rather than its permanent destruction; our experience confirms that in intermediate‐risk patients with persistent AVB it is worth waiting a few days for the inflammation to subside and for intraventricular conduction to be re‐established, in which case there will be no need for a permanent pacemaker.
Other possible complications are related to vascular access and intravascular catheter manipulation, similar to percutaneous coronary intervention, but they are uncommon if care is taken with technical aspects of the procedure. Alam et al. described dissection of the anterior descending artery in 1.8% of patients,16 while cardiac tamponade caused by insertion of the temporary pacing catheter has a reported incidence of 0.6%.16
Alcohol leakage to the anterior descending artery or via collaterals to remote tissues is a serious complication that can lead to an unconfined infarction outside the target area. Leakage can be avoided by taking great care to keep the balloon fully inflated and to maintain the stability of the entire system to prevent reflux of alcohol. The recruitment of collaterals is an unpredictable complication, since as in our experience, they may not be visualized at the beginning of the intervention, but this is rare. In one of the few published cases, Chowdhary et al. suggest that recruitment of collaterals may be facilitated by longer occlusion of the target vessel before alcohol administration,26 which was in fact the case in our patient due to the need to replace the echocardiographic probe. Since then, we have paid particular attention to the need to minimize occlusion time.
Another possible cause for concern is the creation of an intramyocardial septal scar, due to its arrhythmogenic potential in a disease in which there is already a substrate for arrhythmias. However, after nearly 20 years of experience and thousands of procedures, this fear has proved groundless (see following section). Careful stratification of arrhythmic risk is essential, as always in the treatment of HCM.
In‐hospital mortality associated with ASA is 0–4%,27 lower in centers with greater experience with ASA and percutaneous interventions generally, reflecting the need for a learning period. Kwon et al. reported no intraprocedural mortality in patients at high surgical risk.28 In the review by Alam et al., early (30‐day) mortality was 1.5% (0.0–5.0%) and late mortality (>30 days) was 0.5% (0.0–9.3%).16
Comparison with myectomyAs with other conditions for which there are both percutaneous and surgical treatment options, either as alternatives or complementary, there has been considerable debate concerning the best approach. It is generally agreed that there is unlikely to be a large randomized multicenter trial comparing the two strategies that could help clarify which of the two should be preferred.29 The low prevalence of OHCM and the lack of financial incentive (no specialized technology or intellectual property is involved) would make such a trial difficult to implement. We are thus left with comparing the limitations of each technique and the results of observational studies in meta‐analyses.
Proponents of surgery frequently point to the greater long‐term experience with myectomy, although this argument is becoming weaker as the years pass; there are in fact now more patients treated percutaneously than surgically. Surgical treatment can deal with certain anatomical features that ASA cannot, such as mitral valve repair and cases requiring a larger quantity of hypertrophied muscle to be resected, thus achieving a greater and more rapid reduction in intraventricular gradient, but this does not appear to affect clinical outcomes. Complete sustained AVB is less frequent with myectomy (5% as opposed to 10% with ASA); this is probably the main difference in the results of the two techniques.16
ASA has the advantages of other percutaneous treatments: it is less invasive, does not require sternotomy, is associated with shorter recovery time, and can be used to treat patients with relative or absolute contraindications for surgery, such as elderly or obese individuals and those with significant comorbidities. Clinical outcomes are similar to those of myectomy, as shown in two recent meta‐analyses, in which the only difference was the need for permanent pacing.30,31 These results reflect the learning curve required for ASA, as was initially seen for myectomy, although not in recent publications.
Two of the main limitations of ASA are the need for favorable anatomical conditions and more unpredictable outcomes, although these can be reduced by careful patient selection.
The greatest controversy concerns the risk of ventricular arrhythmias with ASA. Such fears were heightened by some small observational studies,32,33 but these were contradicted by other similar studies that indicated the technique was safe.34,35 These conflicting results, and the lack of sufficient evidence, have created confusion that can only be resolved by large studies of patients treated by ASA with long‐term outcomes and by meta‐analyses comparing the results of the two techniques. In 2008 Kuhn et al. published the results of 644 consecutive patients treated by ASA over a 10‐year period.36 Annual cardiac mortality after discharge was 0.7%, a similar percentage to the untreated population, which does not support the idea that these patients are at greater risk of sudden death.37 The latest and largest meta‐analysis of the two techniques analyzed 2207 patients treated by ASA and 1887 patients treated by myectomy and found no differences in overall mortality or sudden death, even though the population treated surgically were younger.38
ConclusionThe results from the initial experience of our center confirm the efficacy and safety of ASA for the invasive treatment of patients with OHCM refractory to medical therapy. This percutaneous approach is a valid alternative for invasive treatment to reduce subaortic obstruction in these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fiarresga A, Cacela D, Galrinho A, et al. Ablação septal alcoólica no tratamento da cardiomiopatia hipertrófica obstrutiva experiência de quatro anos de um centro. Rev Port Cardiol. 2014;33:1–10.