The authors describe a case of a patient admitted to the emergency department with diabetic ketoacidosis. Although there were no symptoms attributable to the cardiovascular system, lab tests revealed elevated troponin I and natriuretic peptides, coupled with repolarization abnormalities on the ECG. The transthoracic echocardiogram (TTE) showed a non-dilated left ventricle with severe left ventricular systolic dysfunction due to diffuse hypokinesia, and a concomitant diagnosis of profile L heart failure was proposed. Etiologic investigation was negative, and when a new TTE was performed seven days after the first, left ventricular function was normal. Although rarely considered, metabolic and electrolyte disorders, especially diabetic ketoacidosis, can be a cause of left ventricular systolic dysfunction, and should be considered in the differential diagnosis. This is another way diabetes can have an impact on the cardiovascular system.
Os autores descrevem um caso de um doente admitido no Serviço de Urgência por cetoacidose diabética. Apesar de não haver queixas do foro cardiovascular, a avaliação analítica revelou uma elevação do nível de troponina I (TropI) e de péptidos natriuréticos (proBNP), associada a alterações da repolarização no eletrocardiograma. O ecocardiograma transtorácico (ETT) mostrou um ventrículo esquerdo não dilatado com disfunção sistólica ventricular esquerda grave, por hipocinesia difusa, assumindo‐se assim concomitantemente um perfil L de insuficiência cardíaca (IC). A investigação etiológica foi negativa e quando um novo ETT foi realizado, sete dias após o primeiro, a função ventricular esquerda era normal. Apesar de raramente considerados, os distúrbios metabólicos e hidroeletrolíticos, nomeadamente a cetoacidose diabética, podem ser uma causa de disfunção VE e esta é outra das formas pela qual a diabetes pode ter impacto no sistema cardiovascular.
The prevalence of diabetes is increasing consistently worldwide. In Portugal it is estimated at 13.3%, of whom 44% are undiagnosed. Furthermore, macrovascular disease, including stroke and myocardial infarction, account for a significant proportion of the morbidity and mortality associated with the disease.1 Diabetes affects the cardiovascular system, particularly the heart, in various ways, the most obvious being coronary artery disease (CAD) in its different manifestations of ischemic heart disease and subsequent left ventricular dysfunction and heart failure (HF). However, in recent years researchers have reported that even in the absence of CAD, patients with diabetes have a higher prevalence of HF, and that HF in these patients, irrespective of its etiology, carries a worse prognosis.2 It is also important to note the relatively new and as yet poorly understood entity known as diabetic cardiomyopathy, in which ventricular dysfunction is caused by oxidative stress induced by hyperglycemia, hyperlipidemia, hypertension and inflammation resulting in abnormal gene expression and the activation of pathways leading to programmed myocardial cell death.3
The present case report aims to show that diabetes has acute as well as long-term cardiovascular effects, and that associated electrolyte, acid-base and hemodynamic changes can mimic the cardiovascular alterations that occur over the course of the disease’s evolution.
Case reportA 46-year-old man was admitted to the emergency department (ED) due to uncontrollable vomiting and altered consciousness (confusion) lasting for two hours. His personal history included type 1 diabetes, diagnosed eight years previously, under insulin therapy but with poor metabolic control, being followed in the diabetes clinic, and an unspecified psychiatric disorder, previously followed in psychiatric consultations. He was a current smoker (70 pack-years) but denied taking any drugs other than insulin therapy, alcohol consumption, or illegal drug use.
At first medical contact, it was noted that the patient had ketone breath and was hypotensive (non-invasive arm blood pressure measurement of 77/52 mmHg), tachycardic (heart rate [HR] 102 bpm), polypneic (respiratory rate 30 cpm), and hypothermic (axillary body temperature 35.8 °C). Capillary testing revealed unmeasurable blood glucose (HIGH test result) and increased ketonemia (3; normal <1.2). Arterial blood gas analysis demonstrated severe metabolic acidosis (pH 7.1, pCO2 9.2 mmHg, pO2 139 mmHg, HCO3- 2.9 mmol/l), hyperlactacidemia (lactates 9 mmol/l), hyperkalemia (K+ 7.52 mEq/l) and hyponatremia (Na+ 126 mEq/l) considered pseudohyponatremia and when corrected for blood glucose estimated to be 500 mg/dl (HIGH capillary test result), giving a figure of 136 mEq/l. A diagnosis of diabetic ketoacidosis (DKA) was made and therapy was begun with rapid intravenous (IV) insulin in continuous perfusion and IV hydration with sodium chloride 0.9% at 100 cc/hour and external warming. The decompensating factor was failure to administer insulin in the previous two days.
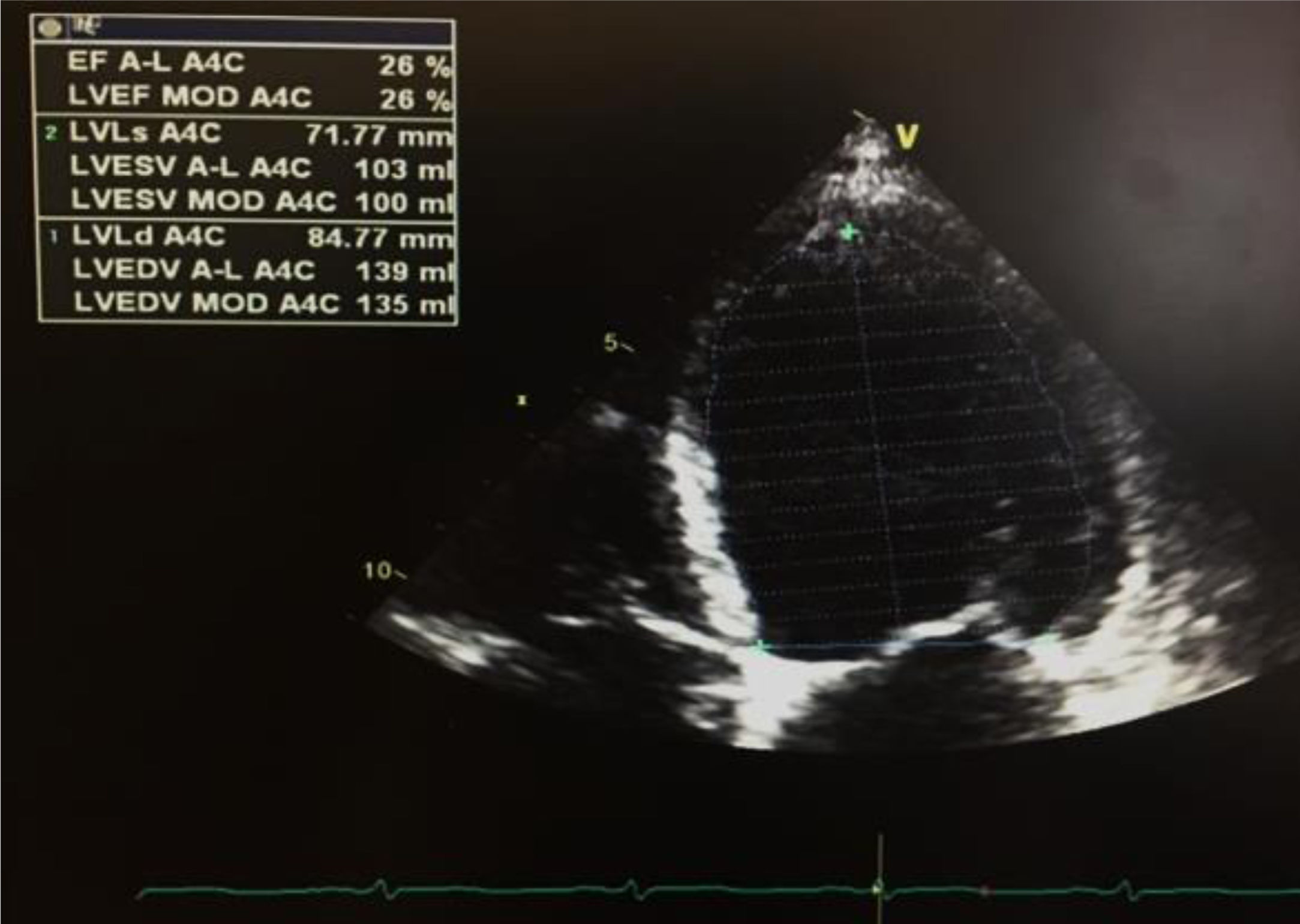
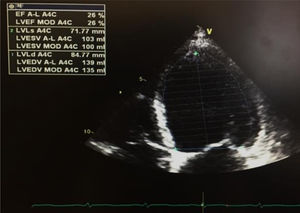
The first venous blood analysis showed elevated troponin I (2.01 ng/ml; normal <0.02 ng/ml), with a peak of 3.23 ng/ml 12 hours after admission. N-terminal pro-brain natriuretic peptide (NT-proBNP) was 17316 pg/ml (normal <125). The patient reported no current or previous chest pain or any symptoms of HF. Physical examination showed no signs of cardiovascular disease; there were no murmurs or extra heart sounds on cardiac auscultation, no rales on pulmonary auscultation, no enlarged organs on abdominal examination and no lower limb edema. The electrocardiogram showed sinus tachycardia (HR 109 bpm) with ST-segment flattening in V5, V6, I and aVL. A transthoracic echocardiogram (TTE) performed on the day of admission using a GE Vivid I portable ultrasound machine revealed a non-dilated and non-hypertrophied left ventricle with severe left ventricular systolic dysfunction (ejection fraction [EF] estimated at 26%) due to diffuse hypokinesia; no significant aortic or mitral valve disease; preserved right ventricular longitudinal systolic function; inferior vena cava 17 mm with preserved respiratory kinetics and pulmonary artery pressure estimated at 28 mmHg (Figure 1).
At 48 hours of hospitalization, and with his DKA resolved, the patient was transferred to the cardiology ward to investigate the etiology of his left ventricular dysfunction. During the investigation it transpired that he had been hospitalized about 15 months before, also with a setting of diabetic ketacidosis. At that time troponin I was also elevated and a rapid TTE performed in the ED demonstrated severe global left ventricular systolic dysfunction, but a second TTE performed in the echocardiographic laboratory eight days after admission was normal. It was assumed that the first exam had been incorrectly reported and no follow-up was scheduled.
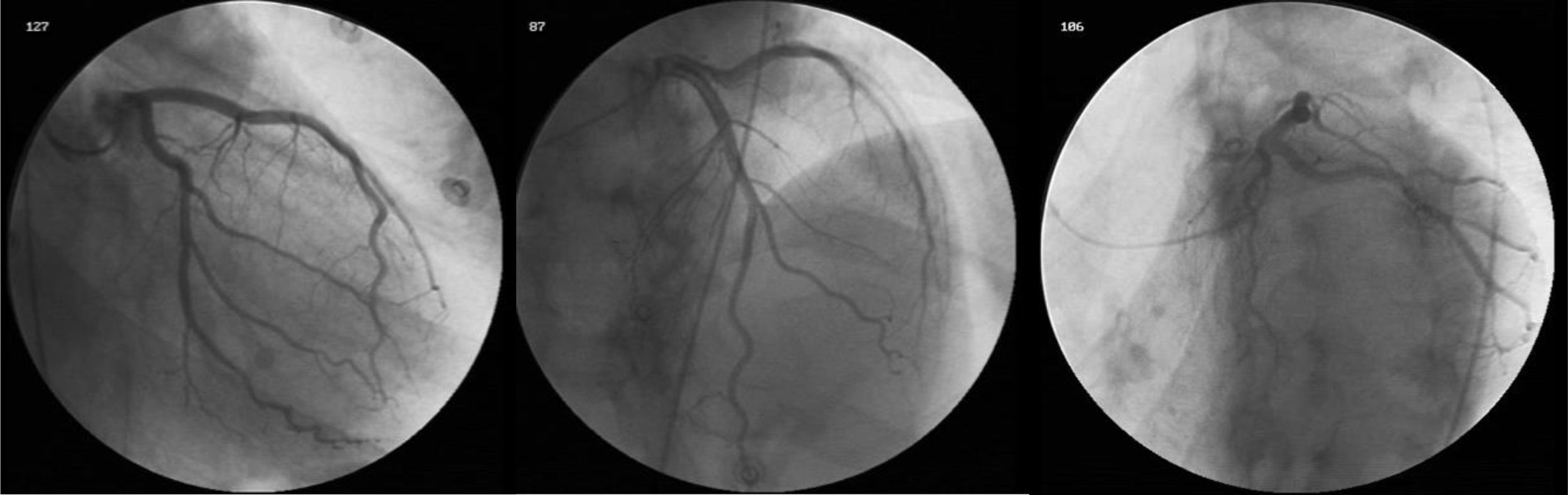
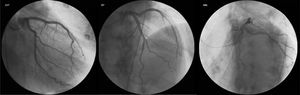
During the patient’s stay in the cardiology ward, diagnostic cardiac catheterization revealed coronary arteries without lesions (Figure 2). There was no history of HF, cardiomyopathy or sudden death, and the patient denied recent viral or bacterial infection and had never traveled abroad. Laboratory tests were negative, including for human immunodeficiency virus and hepatotropic viruses. Thyroid function tests were normal, as they had been throughout his follow-up in the diabetes clinic, although antithyroid antibodies were not assessed. Other laboratory parameters measured during hospital stay included hemoglobin 15 g/dl at admission (nadir 13 g/dl), no blood loss being documented; no functional or absolute iron deficiency (ferritin 326 ng/ml) and no folic acid or vitamin B12 deficiency (5.9 ng/ml and 590 pg/ml, respectively); and vitamin B1, routinely assessed in patients with HF or left ventricular dysfunction, 28 ng/ml (normal 16–48).
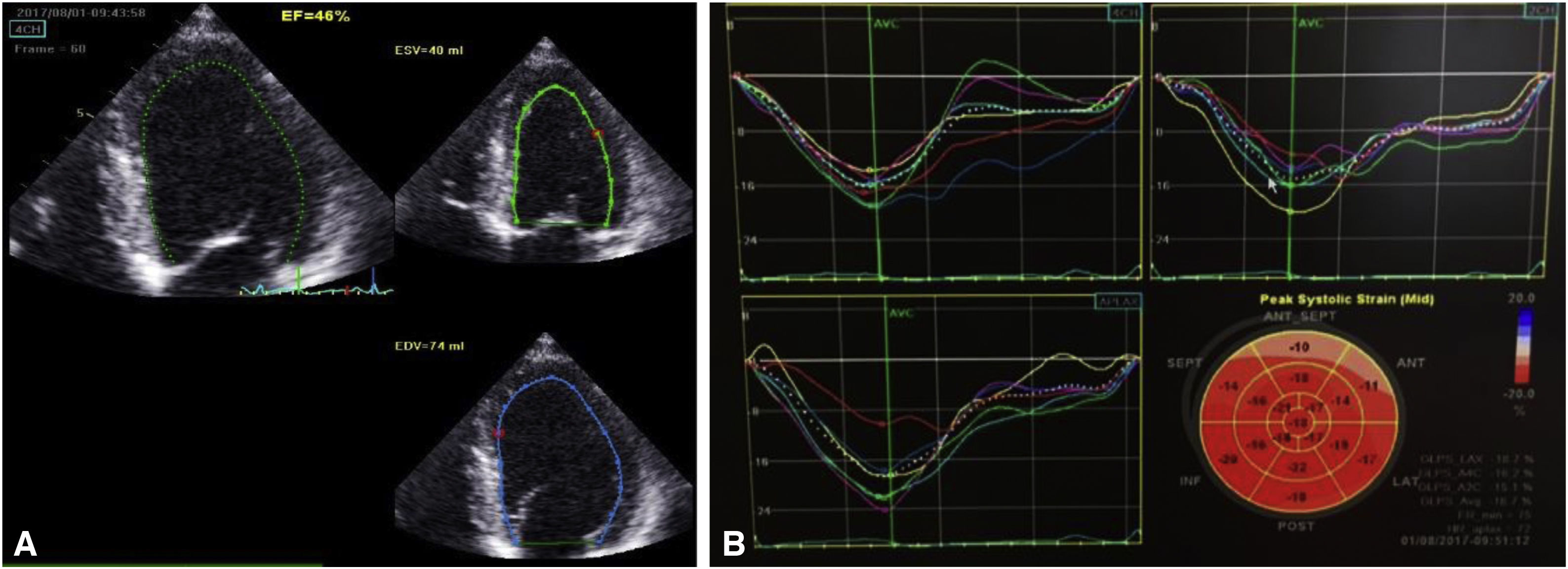
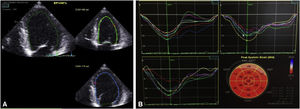
Seven days after admission, a new TTE showed a non-dilated left ventricle, with mild systolic dysfunction (estimated EF 46%) and slightly reduced global longitudinal strain (−16%) (Figure 3). Cardiac magnetic resonance was proposed but the patient refused. At hospital discharge he was in New York Heart Association class I, with NT-proBNP 448 pg/ml, medicated with ramipril 2.5 mg/day and bisoprolol 5 mg/day, as well as slow-release insulin administered twice a day, and was referred for cardiology and diabetes consultations.
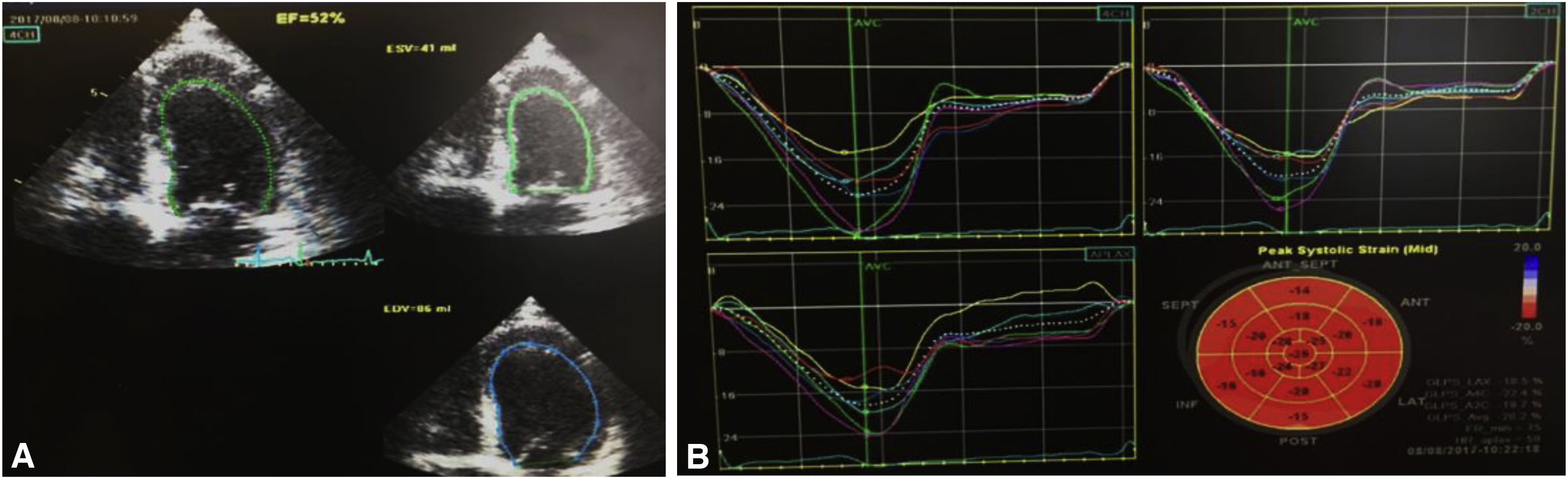
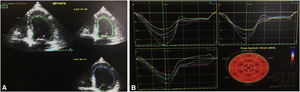
A third TTE, scheduled for one week after discharge, showed preserved global left ventricular systolic function (EF 52%) and normal global longitudinal strain (−20.2%) (Figure 4). At eight months of follow-up, the patient had not been readmitted and had no cardiovascular symptoms.
DiscussionDKA is characterized by the triad of high blood glucose, ketosis and metabolic acidemia resulting from a relative or absolute deficiency of insulin and excess of counter-regulatory hormones, associated with severe electrolyte abnormalities. Patients with DKA usually have mild to moderate hyperkalemia on initial assessment, despite total-body potassium deficiency.
According to international guidelines,4 our patient presented moderate to severe DKA (pH 7.1, HCO3- 2.3 and altered mental status). Thanks to rapid initiation of recommended treatment, including insulin therapy and IV hydration, no life-threatening complications occurred, even though mortality in this entity ranges between 1% and 5% in different registries, depending on age and comorbidities.5 Although our patient presented hyperkalemia, no specific treatment was instituted for this condition, in view of the knowledge that these patients in fact have a total-body K+ deficiency and that beginning IV insulin therapy causes an inflow of K+ into cells, thereby reducing kalemia.
In 2004 Stentz et al. published a study6 aiming to analyze the status of proinflammatory cytokines and markers of oxidative stress and cardiovascular risks associated with the known proinflammatory states of acute and chronic hyperglycemia, in both DKA and nonketotic hyperglycemia. They concluded that both conditions are associated with elevation of proinflammatory cytokines, reactive oxygen species (ROS), and cardiovascular risk factors, including C-reactive protein, homocysteine and plasminogen activator inhibitor-1. They also showed that values of these parameters returned to normal levels (in the absence of obvious infection or cardiovascular pathology) with insulin therapy, and therefore characterize these two conditions as inflammatory diseases.6 Evidence from other studies reveals a significant and independent link between inflammation, sepsis, insulin resistance and cardiac dysfunction.7 The classic outcome of this relationship is systemic inflammatory response syndrome (SIRS), which is found in many conditions, the best-known being sepsis, but also in non-infectious acute inflammatory settings such as acute pancreatitis, trauma and burns. It is defined by the presence of two or more of the following criteria: temperature >38 °C or <36 °C, HR > 90 bpm, respiratory rate >20 cpm or PaCO2 <32 mmHg, and white blood cell count >12 000/mm3 or <4000/mm3 or >10% immature bands.8 Our patient thus fulfilled criteria for SIRS in the setting of acute inflammation due to acute decompensation of diabetes (DKA).
Myocardial dysfunction is a common complication in patients with SIRS due to sepsis and is associated with increased mortality, which can reach 70-90%,8 as well as in SIRS due to trauma or burns.7
The initial TTE in the case presented was performed urgently but after intensive hydration as part of the routine treatment of DKA. As a consequence, it was not possible to estimate the patient’s volemia via the inferior vena cava. We consider that, in contrast to other patients with severe left ventricular dysfunction, in this case such a significant increase in preload caused by intensive hydration to treat DKA might have led to volume overload and acute pulmonary edema. However, this did not occur, probably because besides DKA, the patient also had L-profile HF (‘cold-dry’),9 as shown by hypotension and hyperlactacidemia, markers of hypoperfusion (which may also have contributed to his altered mental state on admission). L-profile HF is most often associated with excessive diuretic use, and the indicated treatment is fluid replacement,9 which in our opinion should be carefully monitored both clinically and by imaging studies such as are readily available in the ED, like echocardiography, in order to prevent complications associated with excessive restoration of volemia.
Berk et al.10 report a case of a patient admitted in 2015 with hyperosmolar hyperglycemic state (HHS), whose TTE performed due to electrocardiographic alterations showed severe left ventricular dysfunction. On the fourth day of hospitalization she underwent imaging tests for ischemia, which were negative and left ventricular function was also normal. The authors suggested as possible causes for this reversible dysfunction HHS, SIRS and euthyroid sick syndrome.10 Nanda et al.11 documented a unique case of DKA-induced stress-related cardiomyopathy in a woman with type 1 diabetes. They hypothesize that the rarity of this association may be due to the fact that myocytes have a decreased ability to metabolize glucose and free fatty acids in takotsubo cardiomyopathy and can preferentially change their metabolic substrate to ketones, which are found in DKA. Furthermore, the association of DKA and stress cardiomyopathy may lead to severe acidemia, with HCO3- 4 mmol/l, which may be one factor in the left ventricular dysfunction reported in this case.11
Besides the role of proinflammatory cytokines and ROS in SIRS-related myocardial dysfunction, changes in calcium homeostasis are also involved, as calcium inflow into myocytes is reduced and release of this cation from the sarcoplasmic reticulum is inhibited.12
The possibility cannot be excluded that the metabolic acidemia found in DKA may also have been a factor in the reversible left ventricular dysfunction observed in the present case. To the best of our knowledge, there have been few reports in the literature on the impact of acid-base disorders on cardiac function. In a 1990 study, Teplinsky et al.14 published an experimental study of the effect of lactic acidosis on hemodynamics and left ventricular function in dogs. They demonstrated that during progressive acidemia induced by continuous IV infusion of lactic acid, cardiac output, stroke volume, and mean systemic arterial pressure fell, while mean pulmonary artery pressure and right atrial pressure increased. The authors concluded that lactic acidemia caused a 40% reduction in stroke volume, which could be attributed to depressed LV contractility, characterized by a decrease in maximum dP/dt.13 Also in 1990, a review of the effects of acidosis on cardiac contractility concluded that this acid-base disorder affects every step in the excitation-contraction coupling pathway, including the counter-regulatory effects on Ca2+ delivery to myofilaments (reduced delivery by inhibition of the Ca2+ current and reduced release of this cation from the sarcoplasmic reticulum, as well as increased delivery by prolongation of the action potential) and the responsiveness of myofilaments to Ca2+, which overall is reduced.14
Another review, published in 1995,15 concluded that the effect of acidemia on ventricular function depends on pH level: in mild acidemia, increased catecholamine release compensates for the cardiac depressant effects of acidemia, with increased inotropy, chronotropy, cardiac output and peripheral vascular resistance, while when pH is <7.2, as in our patient, H+ ions have a direct cardiac depressant action that cannot be compensated by increased catecholamines.15
ConclusionMyocardial dysfunction is a frequent complication associated with SIRS, whether secondary to sepsis or to other acute inflammatory states such as DKA, the common mechanism being high levels of proinflammatory cytokines and ROS. As well as SIRS, other electrolyte and acid-base disorders may have contributed to the reversible left ventricular dysfunction observed in our patient, in whom acute L-profile HF was associated with DKA. We consider that, as the negative impact of ventricular dysfunction on the prognosis of these patients is now proven, echocardiographic assessment should be performed routinely and that those with systolic or diastolic dysfunction should be scheduled for follow-up to monitor reversal of dysfunction, control other risk factors and investigate other concomitant etiologies.
As the pathogenesis of diabetic cardiomyopathy includes oxidative stress,3 the association of the latter with ventricular dysfunction in settings of acute decompensation may point the way to therapeutic studies based on inhibiting these proinflammatory processes, with a view to reducing mortality in these acute settings and hopefully preventing progression to irreversible ventricular dysfunction.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Roque D, Faria D, Ferreira J, Ferreira H, Beringuilho M, Magno P, et al., Uma causa reversível de disfunção ventricular esquerda: caso clínico e breve revisão. Rev Port Cardiol. 2021;40:383–388.