There are conflicting data regarding the clinical benefits of device-based remote monitoring (RM). We sought to assess the effect of device-based RM on long-term clinical outcomes in recipients of implantable cardioverter-defibrillators (ICDs).
MethodsWe assessed the incidence of adverse cardiac events, overall mortality and device therapy efficacy and safety in a propensity score-matched cohort of patients under RM compared to patients under conventional follow-up. Data on hospitalizations, mortality and cause of death were systematically assessed using a nationwide healthcare platform. The primary outcome was time to a composite outcome of first hospital admission for heart failure or cardiovascular death.
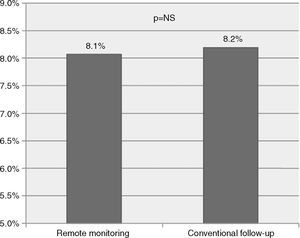
ResultsOf a total of 923 implantable device recipients, 164 matched patients were identified (84 under RM, 84 under conventional follow-up). The mean follow-up was 44 months (range 1-123). There were no significant differences regarding baseline characteristics in the matched cohorts. Patients under RM had a significantly lower incidence of the primary outcome (hazard ratio [HR] 0.42, confidence interval [CI] 0.20-0.88, p=0.022); there was a non-significant trend towards lower overall mortality (HR 0.53, CI 0.27-1.04, p=0.066). No significant differences between cohorts were found regarding appropriate therapies (RM vs. conventional follow-up, 8.1 vs. 8.2%, p=NS) or inappropriate therapies (6.8 vs. 5.0%, p=NS).
ConclusionIn a propensity score-matched cohort of ICD recipients with long-term follow-up, RM was associated with a lower rate of a combined endpoint of hospital admission for heart failure or cardiovascular death.
Existem dados contraditórios acerca dos benefícios clínicos da monitorização remota (MR). Os autores procuraram aferir o efeito da MR de dispositivos cardíacos implantáveis em eventos clínicos a longo prazo.
MétodosOs autores avaliaram a incidência de eventos cardíacos adversos, mortalidade global, e a eficácia e segurança das terapêuticas entregues pelo dispositivo numa amostra de pacientes portadores de cardioversor-desfibrilhador implantável (CDI) após emparelhamento por propensity score, comparando doentes sob MR com doentes em seguimento convencional. Dados relativos a hospitalizações, mortalidade e causa de morte foram sistematicamente avaliados com recurso à Plataforma de Dados de Saúde. A análise primária consistiu no tempo até um evento composto de internamento por insuficiência cardíaca (IC) ou morte cardiovascular.
ResultadosNum total de 923 portadores de dispositivos cardíacos, identificámos 164 pacientes emparelhados (84 sob MR, 84 sob seguimento convencional. O tempo médio de seguimento foi 44 meses (entre 1-123 meses). Não se observaram diferenças significativas nas características basais entre os dois grupos após emparelhamento por propensity score. Doentes sob MR tiveram uma incidência significativamente menor do evento composto de internamento por IC ou morte de causa cardiovascular (hazard ratio [HR] 0,42; intervalo de confiança [IC] 0,20-0,88; p=0,022); houve uma tendência não estatisticamente significativa para uma menor mortalidade global (HR 0,53; IC 0,27-1,04; p=0,066). Não se observaram diferenças entre grupos relativamente a terapêuticas apropriadas (MR versus seguimento convencional; 8,1 versus 8,2%, p=NS) ou terapêuticas inapropriadas (6,8 versus 5,0%, p=NS).
ConclusãoNuma amostra emparelhada por propensity score de portadores de CDI, a MR associou-se a uma menor incidência de um evento composto de internamento por IC ou morte cardiovascular a longo prazo.
Technological developments and expanded indications have resulted in a large population of recipients of implantable electronic cardiac devices, including a significant increase in the number of patients with implantable cardioverter-defibrillators (ICDs). This growing population represents a unique challenge regarding follow-up, requiring an experienced team with in-depth knowledge of device programming and potential complications.1 To date, optimal clinical resource allocation is not established regarding appropriate follow-up for these patients.
In this context, remote monitoring (RM) is poised to be a valuable tool in the intensive and continuous follow-up of ICD patients. Published data support the safety and efficacy of this intervention.1 However, data regarding potential clinical benefits are scarce, with short follow-up periods and mainly limited to specific ICD brands.
In this study we aimed to assess the long-term clinical benefits of RM in a population of ICD patients for primary prevention of sudden cardiac death. To this end, we sought to investigate the effect of RM on hospital admissions for heart failure (HF) and cardiovascular death.
MethodsStudy populationWe performed a propensity-matched retrospective cohort study of consecutive patients referred to a tertiary center for implantation of an ICD for primary prevention who underwent implantation between December 2002 and October 2014.
Implantable cardioverter-defibrillator implantationImplantation of an ICD was performed according to international guidelines2 for patients with systolic dysfunction or primary channelopathies. Implantation in the setting of hypertrophic cardiomyopathy was performed according to disease-specific guidelines.3 Patients indicated for implantation for secondary prevention or with concurrent implantation of a cardiac resynchronization therapy (CRT) device were excluded. ICD patients who underwent an upgrade to a CRT system during follow-up were censored for analysis after the procedure.
Remote monitoring and conventional follow-upRM was offered to patients whenever available as offered by the device manufacturer, with the patient's informed consent. The equipment was provided free of charge at the first outpatient visit after ICD implantation. Patients were then scheduled for a hospital visit every 12 months, with transmissions performed every three months during the study period. Reports were reviewed by trained technicians who would alert the attending physician if there were relevant events. Additionally, RM alerts were transmitted in response to abnormal events such as delivered shocks, detection of atrial fibrillation episodes or abnormal values of the various ICD parameters. In this case, patients were summoned in the following 48 hours for an in-person consultation with an electrophysiologist in order to review the episode.
Conventional follow-up consisted of a patient visit one month after implantation and every four months thereafter. Device interrogation was performed at all visits, and relevant events (e.g. changes in lead impedance or other parameters, detection of atrial fibrillation or appropriate or inappropriate therapies) were recorded. The patient was then observed by an electrophysiologist, and changes in device programming, reintervention, drug therapy or indication for ablation were employed as deemed appropriate.
Data collectionData on arrhythmic events, device programming and appropriate and inappropriate ICD therapies were prospectively entered into a database consisting of all patients. These records were then assessed at the time of this study for possible missing data and completed whenever possible. Patients were considered lost to follow-up when the last outpatient visit was >18 months previously with no further scheduled visits.
Propensity score matchingTo control for the nonrandom assignment of patients, a logistic regression model was constructed that predicted the likelihood that a patient would be under RM and matched patients in each cohort by this score. The explanatory variables used were baseline characteristics including age, gender, etiology of cardiac disease, left ventricular ejection fraction (LVEF) and New York Heart Association (NYHA) class. To ensure close matches, the difference in the calculated propensity score was required to be below 0.005 between paired subjects.
Primary outcomeThe primary endpoint was time to a composite endpoint of first hospital admission for HF or cardiovascular death. Information on hospitalizations and mortality was accessed via a nationwide healthcare platform (Plataforma de Dados de Saúde) that holds the records of nine out of 10 of the referring hospitals and the National Registry on Mortality. Patient records were systematically reviewed, and information was collected regarding hospital admissions and all-cause mortality. The cause of hospitalization was determined according to an International Classification of Disease 9 (ICD-9)-based system as coded by the discharging hospital. Likewise, cause of mortality was determined according to the same ICD-9-based coding. The physician responsible for assessing study outcomes was blinded to the modality of follow-up (RM vs. in-office).
In addition, the incidence of appropriate and inappropriate device therapies was compared between the matched cohorts.
Statistical analysisContinuous variables were expressed as mean and standard deviation when they followed a normal distribution, and as median and interquartile range otherwise. Qualitative variables were expressed as frequency and percentage. Baseline comparisons were performed using the chi-square test for qualitative data and the Student's t test for continuous variables for unmatched subjects and for differences between groups in matched pairs.
Analysis of the primary outcome of time to admission for HF or cardiovascular death was performed by Kaplan-Meier curves, the log-rank test for comparisons and a Cox proportional hazards model for calculation of the hazard ratio (HR). A two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using the software package STATA 12 (Statacorp).
ResultsStudy populationWe identified a total of 923 patients who received an ICD during this period. Of these, 611 patients were excluded on the basis of concomitant CRT (n=473) or implantation in the setting of secondary prevention (n=138). Patients who were under RM were more frequently male, older, had a lower LVEF, and were more likely to have an ischemic etiology.
After propensity score matching, we identified 168 patients corresponding to 84 matched pairs who remained in the study population. There were no significant differences regarding clinically significant baseline characteristics between the two cohorts. Baseline characteristics after propensity score matching are presented in Table 1.
Baseline characteristics after propensity score matching.
| Remote monitoring | Standard care | p | |
|---|---|---|---|
| No. | 84 (50%) | 84 (50%) | |
| Age at implantation (years) | 60.1±11.3 | 59.7±13.4 | NS |
| Male | 73 (86.9%) | 74 (88.1%) | NS |
| LVEF (%) | 28.9±9.7 | 28.0±8.8 | NS |
| Etiology | NS | ||
| Ischemic | 53 (63.1%) | 52 (61.9%) | |
| DCM | 24 (28.6%) | 25 (29.8%) | |
| HCM | 1 (1.2%) | 5 (5.9%) | |
| Brugada syndrome | 2 (2.4%) | 1 (1.2%) | |
| Other | 3 (3.6%) | 1 (1.2%) | |
| NYHA class | NS | ||
| I | 11 (13.1%) | 10 (11.9%) | |
| II | 60 (71.4%) | 63 (75.9%) | |
| III | 13 (15.5%) | 11 (13.1%) | |
| IV | 0 (0%) | 0 (0%) | |
| Device brand | <0.001 | ||
| Biotronik | 39 (46.4%) | 4 (4.7%) | |
| Boston Scientific | 7 (8.3%) | 18 (21.2%) | |
| Medtronic | 18 (21.4%) | 29 (34.1%) | |
| Sorin | 18 (21.4%) | 22 (25.9%) | |
| St. Jude Medical | 2 (2.4%) | 11 (12.9%) | |
| Device type | |||
| Single chamber | 80 (95.2%) | 79 (94.0%) | |
| Dual chamber | 4 (4.8%) | 5 (6.0%) | |
DCM: dilated cardiomyopathy; HCM: hypertrophic cardiomyopathy; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association.
Mean LVEF was 28.4%, with no difference between groups (RM vs. controls 28.9±9.6% vs. 28.0±8.8%, p=NS). No significant differences were found regarding age at implantation (56.9±1.3 vs. 57.9±1.1 years, p=NS).
Most patients had an indication for ICD in primary prevention in the setting of left ventricular systolic dysfunction (91.7%). The etiology was ischemic cardiomyopathy in 62.5%, idiopathic dilated cardiomyopathy in 29.2%, hypertrophic cardiomyopathy in 3.6%, Brugada syndrome in 1.8% and other etiologies in 3.0% (one patient with hypertensive heart disease, one with congenital heart disease, two with valvular heart disease and one with left ventricular non-compaction cardiomyopathy).
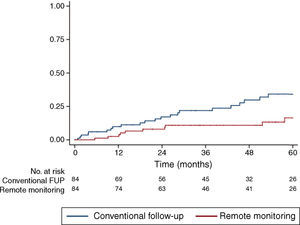
Follow-upMean follow-up was 44 months (interquartile range 20-66), with no patients lost to follow-up during the 12-year study period. Mean follow-up duration was similar between cohorts (43.0±29.8 months for conventional follow-up vs. 46.1±27.9 months for RM, p=NS). Data on the primary outcome were available for all 168 patients, and were used to calculate the Kaplan-Meier failure estimates.
Study outcomesRM was significantly associated with a lower incidence of the primary outcome of hospital admission for HF or cardiovascular death (HR 0.42, confidence interval [CI] 0.20-0.88, p=0.022). This difference, manifested early during follow-up and increasing throughout the study period, suggests a cumulative benefit of RM with longer follow-up (Figure 1).
In addition, the effect of RM on overall mortality was assessed. Although we did not find a significant difference between groups, there was a trend toward lower mortality in the RM group (HR 0.53, CI 0.27-1.04, p=0.066).
To test whether increased availability of RM in recent years could explain the improved outcomes in these patients, we controlled for the effect of year of implantation by patients into quartiles according to year of implantation. We found no effect of year of implantation on overall survival (log rank, p=0.177) or on the effect of RM on mortality (p for interaction=0.384).
To study the effectiveness and safety of ICD therapies, we analyzed the rate of device therapies (shock or antitachycardia pacing) during the study period. We found no significant difference between the incidence of appropriate device therapies (8.1 vs. 8.2 per 100 person/years, p=NS, Figure 2) or inappropriate therapies (6.8 vs. 5.0 per 100 person/years, p=NS).
DiscussionIn this long-term study of two modalities of device follow-up in ICD recipients, we found a significantly lower rate of a combined endpoint of unplanned hospital admission for HF and cardiovascular mortality in patients under RM. The overall rate of death was not significantly different between cohorts, although there was a numerical trend toward lower overall mortality in patients under RM. In addition, the efficacy and safety of ICD implantation did not differ between groups, with a similar rate of appropriate and inappropriate therapies during the study period.
Our study cohort consisted of unselected all-comers with indication for ICD implantation for primary prevention of sudden cardiac death. To validate our findings and adjust for the potential confounding effect of baseline characteristics, we performed a propensity score-matched analysis which successfully attained a balanced cohort of patients.
As previously stated, there was no formal randomization of patients, with RM instituted according to an independent external factor, in this case the availability of the RM hardware as offered by the device manufacturer. This led to some imbalance in the distribution of device brands between patients under RM or conventional follow-up.
Data were available on hospital admissions and mortality for the entirety of our cohort, and cause of death or hospitalization was assessed as described in the Methods section. Patients were followed for up to 12 years, with a consistent benefit of RM being seen throughout follow-up.
Contemporary wireless RM systems avoid the need for manual wanded transmissions and perform automatic transmissions requiring minimal patient input. Furthermore, there are recognized gains in resource efficiency, and some clinical benefits have also been demonstrated in clinical trials. The mechanisms whereby RM may lead to improved patient outcomes, as assessed by these studies, include better adherence to follow-up and a faster time to physician assessment after event occurrence,4 lower incidence of atrial fibrillation and stroke-related hospital admissions,5 and reduced length of hospital stay. Other benefits include therapy guided by intrathoracic impedance and monitoring of ventricular arrhythmias.
Most studies on RM have been underpowered to effectively assess clinical outcomes, due to small numbers of recruited patients or short duration of follow-up. Recently, the PREDICT-RM trial demonstrated reductions in all-cause rehospitalization and all-cause mortality in a large cohort of patients after new ICD implantation who underwent RM during follow-up.6 In the IN-TIME study, Hindricks et al.7 randomized 716 patients with left ventricular dysfunction who received an ICD (with or without CRT) to automatic, daily, implant-based telemonitoring vs. conventional follow-up in a single-blinded design. After one year, patients under RM had a lower composite score of all-cause mortality, admission for HF, change in NYHA class, and patient global self-assessment. The secondary outcome of all-cause death was significantly lower in the RM group (10 vs. 27 patients).
The ALTITUDE Survival Study8 was a registry-based study which assessed the long-term survival of 194006 recipients of Boston intracardiac ICD and CRT devices. Patients who were under RM follow-up with transmission of information to the LATITUDE network had significantly improved long-term survival, with a 50% relative risk reduction in overall mortality (HR 0.56 for ICD recipients; HR 0.45 for CRT recipients). In addition, the recently published EFFECT study,9 a prospective, observational trial of RM in patients treated by ICD-CRT implantation, also showed an improvement in the combined endpoint of death or cardiovascular hospitalization.
Not all studies on the effect of RM on mortality have been positive. The TELE-HF trial10 randomized 1653 high-risk patients to telephone telemonitoring or conventional follow-up. The main outcome of all-cause mortality or rehospitalization at 180 days was not significantly improved. However, adherence to and frequency of use of RM may have a significant impact on its clinical effect. A recently published systematic review and meta-analysis11 of clinical outcomes in randomized controlled trials of RM vs. conventional follow-up showed that a survival benefit was observed (odds ratio 0.65; p=0.021) only in the subset of three trials with daily verification of transmission, as is performed in our center. In addition, a graded relationship between adherence to RM and improved survival was observed in a recent ‘big data’ study of 269471 implants by Varma et al.12
In our study, patients with concomitant CRT were excluded. This population could conceivably derive a similar or larger clinical benefit from RM, owning to the added complexity of their outpatient management. However, the effect of CRT on disease history and the variability in echocardiographic and clinical responses to CRT would make it more difficult to ascertain the exact effect of RM in these patients. In our study, patients who were subsequently upgraded to an ICD-CRT were censored from analysis after the procedure to exclude a potential confounding effect.
While the magnitude of the effect of RM on HF admission and CV mortality in our study is surprising, it is similar to previous reports such as the IN-TIME study and the ALTITUDE survival registry. Suggested mechanisms for the mortality benefit observed with RM include earlier notification of adverse events, increased patient involvement in their own healthcare, and a more comprehensive assessment of the cardiac health and arrhythmia status of each patient. In any case, our data support the notion that RM is certainly not harmful, and may have a beneficial long-term impact on overall mortality, which appears to stem mainly from lower cardiovascular mortality and a reduction in the incidence of hospitalizations for HF, a well-recognized adverse prognostic factor.
LimitationsThis was a single-center non-randomized study, and thus the generalizability of our results is limited. As this was a retrospective cohort trial, we cannot exclude the effect of unmeasured confounders or some degree of bias; in particular, although there were no significant differences between the groups after propensity score matching, we cannot exclude the potential effect of indication bias. In addition, since patients had to agree to the use of RM, we could hypothesize that some of those who refused RM also had lower adherence to other measures (such as medication), which could make RM a surrogate for non-adherence. However, the measured patient characteristics were well balanced between groups at baseline, and the magnitude and temporal consistency of the clinical benefit in the RM group suggest a more significant effect than what would be explained by lower compliance. In addition, the use of ‘hard’ endpoints (hospitalization or death) and of a publicly available database reduces the possibility of under-reporting of events in the conventional follow-up group as compared to the RM group.
Finally, our study was designed to assess the impact of device-based RM, as performed in daily practice, on relevant clinical events. However, since there was no systematic assessment of the cause of RM alerts or transmissions, we cannot report on the specific relevance of the different monitored parameters such as thoracic impedance or detection of atrial fibrillation.
ConclusionsIn a propensity score-matched cohort of ICD recipients with long-term follow-up, RM was associated with a lower rate of a composite outcome of hospital admission for HF or cardiovascular death. Larger, appropriately powered randomized clinical trials are warranted to definitely establish whether device-based RM can lead to improved outcomes in this high-risk population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.