To analyze the experience gained in 10 years of the heart transplantation program of the University Hospital of Coimbra.
MethodsBetween November 2003 and December 2013, 258 patients with a mean age of 53.0±12.7 years (3–72 years) and predominantly male (78%) were transplanted. Over a third of patients had ischemic (37.2%) and 36.4% idiopathic cardiomyopathy. The mean age of donors was 34.4±1.3 years and 195 were male (76%), with gender difference between donor and recipient in 32% of cases and ABO disparity (non-identical groups but compatible) in 18%. Harvest was distant in 59% of cases. In all cases total heart transplantation with bicaval anastomoses, modified at this center, was used. Mean ischemia time was 89.7±35.4 minutes. All patients received induction therapy.
ResultsEarly mortality was 4.7% (12 patients) from graft failure and stroke in five patients each, and hyperacute rejection in two. Thirteen patients (5%) required prolonged ventilation, 25 (11.8%) required inotropic support for more than 48 hours, and seven required pacemaker implantation. Mean hospital stay was 15.8±15.3 days (median 12 days). Ninety percent of patients were maintained on triple immunosuppressive therapy including cyclosporine, the remainder receiving tacrolimus. In 23 patients it was necessary to change the immunosuppression protocol due to renal and/or neoplastic complications and humoral rejection. All but two patients have been followed in the Surgical Center. Fifty patients (19.4%) subsequently died from infection (18), cancer (10), vascular (eight), neuropsychiatric (four), cardiac (two) or other causes (eight). Forty-six patients (17.8%) had episodes of cellular rejection (>2 R on the ISHLT classification), eight had humoral rejection (3.1%), and 22 have evidence of graft vascular disease (8.5%). Actuarial survival at 1, 5, and 8 years was 87±2%, 78±3% and 69±4%, respectively.
ConclusionThis 10-year series yielded results equivalent or superior to those of centers with wider and longer experience, and have progressively improved following the introduction of changes prompted by experience. This program has made it possible to raise and maintain the rate of heart transplantation to values above the European average.
Analisar a experiência adquirida em dez anos do programa de transplantação cardíaca dos Hospitais da Universidade de Coimbra.
MétodosDe novembro de 2003 a dezembro de 2013, 258 doentes com idade média de 53,0±12,7 anos (limites 3-72 anos) e predominância do sexo masculino (78%) foram transplantados. Mais de um terço dos doentes tinha miocardiopatia isquémica (37,2%) e 36,4% idiopática. A idade média dos dadores era 34,4±11,3 anos e 195 eram do sexo masculino (76%), com disparidade de sexo entre dador e recetor (F:M) em 32% dos casos e disparidade AB0 (grupos não idênticos mas compatíveis) em 18%. A colheita foi feita à distância em 59% dos casos. Em todos os casos foi utilizada a técnica de transplantação total, com anastomose bicava, modificada neste centro. O tempo médio de isquemia foi 89,7±35,4 minutos. Todos os doentes receberam terapêutica de indução.
ResultadosA mortalidade precoce foi 4,7% (12 doentes), por falência do enxerto e acidente vascular cerebral em cinco cada, e por rejeição hiperaguda em dois. Treze doentes (5%) necessitaram de ventilação prolongada e 25 (11,8%) requereram suporte inotrópico por mais de 48 horas, sete necessitaram de implantação de pacemaker. O tempo médio de internamento foi de 15,8±15,3 dias (mediana, 12 dias). Noventa por cento dos doentes foram mantidos com terapêutica imunossupressora tripla, incluindo ciclosporina. Os restantes receberam tacrolimus. Em 23 doentes foi necessário alterar o esquema de imunossupressão devido a complicações renais e/ou neoplásicas e rejeição humoral. Todos os doentes, exceto dois, são seguidos no centro cirúrgico. Cinquenta doentes (19,4%) faleceram tardiamente por infeção (18 doentes), neoplasia (dez doentes), causa vascular (oito doentes), neuropsiquiátrica (quatro doentes), cardíaca (dois doentes) ou outras (oito doentes). Quarente e seis doentes (17,8%) tiveram episódios de rejeição celular (≥2 R da ISHLT) e oito tiveram rejeição humoral (3,1%), e em 22 há evidência de doença vascular enxerto (8,5%). A sobrevivência atuarial a um, cinco, e oito anos foi de 87±2%, 78±3% e 69±4%, respetivamente.
ConclusãoNesta série de dez anos obtiveram-se resultados equivalentes ou superiores aos referidos em experiências mais vastas e mais longas, progressivamente melhorados pela introdução de fatores suscitados pela própria experiência. Com este programa foi possível elevar e manter a taxa de transplantação cardíaca em valores acima da média europeia.
Cardiac transplantation is the most effective treatment for end-stage heart failure (HF) that is refractory to other therapies, not only increasing survival but improving patients’ quality of life.1–3 However, there has been a marked decrease in the number of donors, which is the main limitation to transplantation. This has led to a widening of the selection criteria in recent years to include older donors and those who have died of neurological causes, frequently with other diseases and/or cardiovascular risk factors.3,4
At the same time, progress in medical treatment of advanced HF has led to improvements in patients’ clinical condition, delaying their entry to the transplantation waiting list. New inotropic and vasodilator agents, cardioverter-defibrillators and resynchronization devices, temporary mechanical circulatory support systems, and the widening of indications for conventional surgery, as well as the establishment of HF intensive care units, have helped to control recurrent HF crises, all of which improve survival but have little effect on patients’ quality of life.5–8 Transplantation thus remains the last resort for patients who do not respond to these new therapies and technologies, and referral rates have not fallen.1,4,6
The cardiac transplantation program at our center began in November 2003. Overall results of the first five years of activity, in terms of morbidity, mortality, survival and quality of life, have been published in a detailed report in the Journal.9 In the present study, we aim to extend this analysis to 10 years and to assess the experience gained.
MethodsPatientsBetween November 2003 and December 2013, a total of 258 patients were transplanted, most of whom (71%) were classified as urgent or emergent (admitted to the HF intensive care unit).
In most cases patients were diagnosed and treated medically in our institution before transplantation. Almost half (126; 48.8%) were referred by institutions outside the normal catchment area of the University Hospital of Coimbra. Following transplantation, immediate and early postoperative care is provided in our center, as is regular medium- and long-term clinical follow-up and treatment of complications. These are the responsibility of the surgical team, which has its own internal medicine specialist. In rare instances care is provided by centers nearer to the patient's place of residence, as is the case with two transplantees currently living abroad.
Cross-matching was performed in all cases and transplantation took place only if the exam was negative. Panel-reactive antibody (PRA) testing was also carried out, the results of which were usually only known after the procedure, and would have influenced the immunosuppression protocol in some cases.
The organ was harvested by members of the transplantation team in all cases. The surgical technique has been previously described, consisting of the standard bicaval anastomosis method used in most transplantation centers, but with small changes prompted by experience, aimed at minimizing ischemia time, particularly through earlier aortic anastomosis and unclamping.
The standard immunosuppressive regimen consists of induction therapy with basiliximab, corticosteroids and oral mycophenolate mofetil immediately before and after the operation, followed by maintenance triple immunosuppressive therapy with corticosteroids, mycophenolate mofetil and calcineurin inhibitors (usually cyclosporine). Doses are adjusted on the basis of a variety of factors, including serum immunosuppressant levels, PRA values, the results of serial endomyocardial biopsies and clinical factors.
Statistical analysisSince the beginning of the program, and with the knowledge and consent of the transplantees, a simultaneous retrospective and prospective analysis has been performed, consisting of collection of clinical data on the pre- and peritransplantation period and on the occurrence of complications in the post-transplant period, using a comprehensive database established jointly with the Portuguese Society of Transplantation.
Continuous variables are presented as means ± standard deviation, and categorical variables are expressed as frequencies and percentages. Survival, overall and by group, was analyzed by the Kaplan-Meier method, and statistical significance was analyzed using the log-rank test. Values of p<0.05 were considered statistically significant.
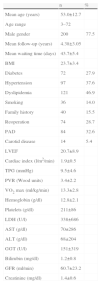
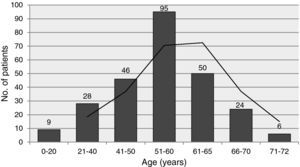
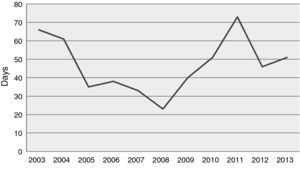
ResultsPatientsThe mean age of the 258 patients was 53.0±12.7 years (3–72 years), predominantly male (77.5%) (Table 1). The age distribution of recipients is shown in Figure 1; most were aged between 40 and 65. Six patients aged over 70 were transplanted. Mean waiting time was 43.7±45.4 days, which has tended to increase in recent years (Figure 2).
Demographic and clinical characteristics of transplanted patients (n=258).
| n | % | |
| Mean age (years) | 53.0±12.7 | |
| Age range | 3–72 | |
| Male gender | 200 | 77.5 |
| Mean follow-up (years) | 4.38±3.05 | |
| Mean waiting time (days) | 43.7±5.4 | |
| BMI | 23.7±3.4 | |
| Diabetes | 72 | 27.9 |
| Hypertension | 97 | 37.6 |
| Dyslipidemia | 121 | 46.9 |
| Smoking | 36 | 14.0 |
| Family history | 40 | 15.5 |
| Reoperation | 74 | 28.7 |
| PAD | 84 | 32.6 |
| Carotid disease | 14 | 5.4 |
| LVEF | 20.7±8.9 | |
| Cardiac index (l/m2/min) | 1.9±0.5 | |
| TPG (mmHg) | 9.5±4.6 | |
| PVR (Wood units) | 3.4±2.2 | |
| VO2 max (ml/kg/min) | 13.3±2.8 | |
| Hemoglobin (g/dl) | 12.8±2.1 | |
| Platelets (g/dl) | 211±86 | |
| LDH (U/l) | 338±686 | |
| AST (g/dl) | 70±286 | |
| ALT (g/dl) | 68±204 | |
| GGT (U/l) | 151±319 | |
| Bilirubin (mg/dl) | 1.2±0.8 | |
| GFR (ml/min) | 60.7±23.2 | |
| Creatinine (mg/dl) | 1.4±0.6 |
ALT: alanine transaminase; AST: aspartate aminotransferase; BMI: body mass index; GFR: glomerular filtration rate; GGT: gamma-glutamyl transferase; LDH: lactic dehydrogenase; LVEF: left ventricular ejection fraction; PAD: peripheral arterial disease; PVR: pulmonary vascular resistance; TPG: transpulmonary gradient; VO2 max: peak oxygen uptake.
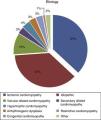
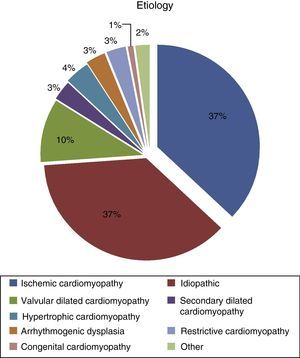
Most patients suffered from ischemic cardiomyopathy (37.2%), followed by idiopathic etiology (36.4%) (Figure 3), and 28.7% had previously undergone cardiac surgery.
Mean body mass index (BMI) at the time of transplantation was 23.7±3.4 kg/m2. Almost a third of patients were diabetic (27.9%) and hypertension (37.6%), dyslipidemia (46.9%) and smoking (14.0%) were observed in significant percentages. Other comorbidities included peripheral arterial disease in 32.6% and carotid disease in 5.4%.
Mean left ventricular ejection fraction was 20.7±8.9%. Pulmonary artery systolic pressure was 48.2±15.3 mmHg, pulmonary vascular resistance 3.4±2.2 Wood units and transpulmonary gradient 9.5±4.6 mmHg. Baseline creatinine was 1.4±0.6 (0.5–5.6) mg/dl, hemoglobin 12.8±2.1 g/l and lactic dehydrogenase 338±686 U/l.
DonorsThe demographic and clinical characteristics of the donors are shown in Table 2. Mean age was 34.4±11.3 years, and most were male (75.6%) and blood group O (53.5%). Cause of death was trauma in most cases (57.8%), followed by hemorrhagic stroke (32.9%), but the number of donors who died of stroke has tended to increase (representing almost 35% of the overall total), and the ratio between trauma and neurological cause of death has now reversed.
Demographic and clinical characteristics of donors (n=258).
| n | % | |
| Mean age, years (maximum) | 34.4±11.3 (57) | |
| Age ≥50 years | 27 | 10.5 |
| Male gender | 195 | 75.6 |
| Gender mismatch | 83 | 32.2 |
| M recipient and F donor | 44 | 17.1 |
| F recipient and M donor | 39 | 15.1 |
| Blood group | ||
| O | 138 | 53.5 |
| A | 113 | 43.8 |
| B | 6 | 2.3 |
| AB | 1 | 0.4 |
| ABO disparity (compatible) | 47 | 18.2 |
| Inotropic support >1 week | 11 | 4.3 |
| Ventilatory support >1 week | 32 | 12.4 |
| Ischemic stroke | 4 | 1.6 |
| Hemorrhagic stroke | 85 | 32.9 |
| Head trauma | 149 | 57.8 |
| Provenance | ||
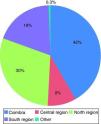
| Coimbra | 107 | 41.5 |
| Central region | 23 | 8.9 |
| North region | 77 | 29.8 |
| South region | 50 | 19.4 |
| Other | 1 | 0.3 |
F: female; M: male.
A significant number of donors underwent prolonged (more than one week) ventilatory or inotropic support (12.4% and 4.3%, respectively).
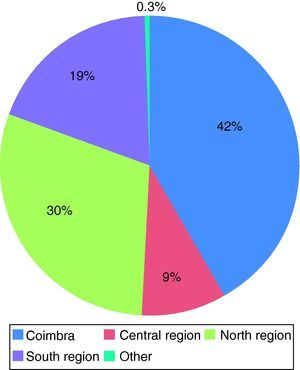
Most organs were harvested in hospitals distant from Coimbra (58.5%), the largest proportion from the North region, which contributed 29.8% of donors; one harvest was from the Azores (Figure 4). Mean distance between donor and recipient was 97.6±100.9 km.
Surgery and intensive careMean ischemia time was 89.7±35.4 minutes and was under 45 minutes in all cases of local harvest. Cardiopulmonary bypass time was 100.3±45.1 minutes. Surgery was also performed on the donor heart in 16 cases (6.2%): mitral valvuloplasty in 12 patients (4.7%), tricuspid annuloplasty in two (0.8%; isolated and associated with mitral repair in one patient each) and revascularization of the anterior descending artery in two patients (0.8%). One patient required replacement of the ascending aorta. Four patients (1.6%) who had been awaiting renal transplantation underwent simultaneous renal and cardiac transplantation (Table 3).
Surgical procedures.
| n | % | |
| Mitral valvuloplasty | 12 | 4.7 |
| Tricuspid annuloplasty | 2 | 0.8 |
| AD revascularization | 2 | 0.8 |
| Renal transplantation | 4 | 1.6 |
| Ischemia time | 89.7±35.4 | |
| CPB time | 100.3±45.1 | |
| MV time (hours) | 20.0±23.8 | |
| Inotropic support >48 hours | 25 | 11.8 |
| Mechanical assistance | 13 | 5.0 |
| Control of hemostasis | 14 | 5.4 |
| Pericardial effusion (within 30 days) | 7 | 2.7 |
AD: anterior descending artery; CPB: cardiopulmonary bypass time; MV: mechanical ventilation.
Most patients were extubated around 12 hours after the operation; mean mechanical ventilation time was 20.0±23.8 hours. Post-transplantation ventricular dysfunction requiring prolonged inotropic support occurred in 25 patients (11.8%), and in 13 of these (5.0%) inotropic support was insufficient and mechanical assistance by intra-aortic balloon pump, left ventricular assist device or extracorporeal membrane oxygenation was used according to circumstances.
Rejection and associated complicationsNinety percent of patients were maintained on triple immunosuppressive therapy including cyclosporine, the remainder receiving tacrolimus in various combinations. In 23 patients it was necessary to change the immunosuppression protocol due to renal and/or neoplastic complications and humoral rejection.
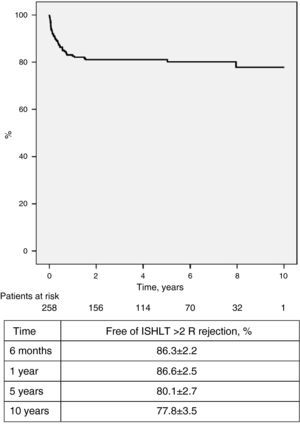
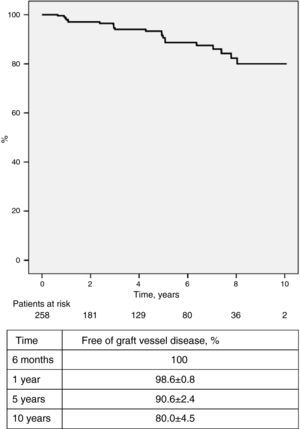
Regular endomyocardial biopsies, echocardiographic studies and angiographic exams were performed to screen for rejection. Forty-six patients (17.8%) had 60 episodes of cellular rejection (>2 R on the International Society of Heart and Lung Transplantation [ISHLT] scale) requiring pulse therapy and/or adjustment of immunosuppressant dosage. One hundred and seven (41.5%) had no episodes of cellular rejection (ISHLT 0 R), while the others had at least one episode of low-grade rejection (1 R) not requiring treatment. The incidence of ≥2 R rejection was highest during the first year after transplantation and tended to decrease progressively in subsequent years; survival free of ≥2 R rejection at six months, one year, five years and 10 years was 86.3±2.2%, 82.6±2.5%, 80.1±2.7% and 77.8±3.5%, respectively (Figure 5). Humoral rejection occurred in eight patients (3.1%). Graft vascular disease (GVD), diagnosed by coronary angiography and defined as any degree of new irregularity of the major epicardial coronary arteries or their main branches, even if <50%, was diagnosed in 22 patients (8.5%), and was mild in most cases. Survival free of GVD at 10 years was 80.0±4.5% (Figure 6).
Other morbidityAll except two patients have been followed in the Surgical Center and complete information is available for all. Mean follow-up was 4.38±3.05 years. Thirty-three patients (12.8%) developed new-onset diabetes after transplantation, 29 of them in the first year following transplantation. Serious infections requiring hospitalization and intravenous antibiotic therapy occurred in 43 patients (16.7%) in the first year. The most frequent infection within six months of the operation was pneumonia (9.7% of patients) (Table 4).
Complications, postoperative and during follow-up.
| n | % | |
| NODAT in the first year | 29 | 11.2 |
| Infections | ||
| Pneumonia | 64 | 24.8 |
| Pneumonia in the first six months | 25 | 9.7 |
| Fungal | 12 | 4.7 |
| CMV | 8 | 3.1 |
| Pyelonephritis | 17 | 6.6 |
| Serious infections within one year | 43 | 16.7 |
| Tumors | ||
| Benign | 18 | 7.8 |
| Malignant | 42 | 16.3 |
| Skin | 20 | 7.8 |
| Prostate | 5 | 1.9 |
| Gastrointestinal | 4 | 1.6 |
| Blood | 4 | 1.6 |
| Brain | 2 | 0.8 |
| Breast | 2 | 0.8 |
| Others | 9 | 3.5 |
| Hemodialysis/renal transplantation | 11 | 4.3 |
| Peripheral vascular disease | 7 | 2.7 |
| Pacemaker implantation | 7 | 2.7 |
CMV: cytomegalovirus; NODAT: new-onset diabetes after transplantation.
Renal function generally recovered within a few months of transplantation and this improvement was maintained during the first year. Only five patients (1.9%) required early renal replacement therapy (≤1 month following transplantation). However, there was progressive and persistent deterioration in renal function after 12 months, as a consequence of immunosuppressive and antibiotic therapy (Table 5). Three patients have undergone renal transplantation, and two more are under renal replacement therapy while awaiting transplantation.
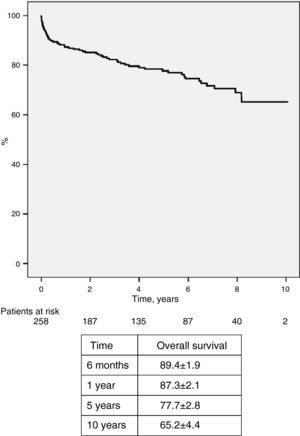
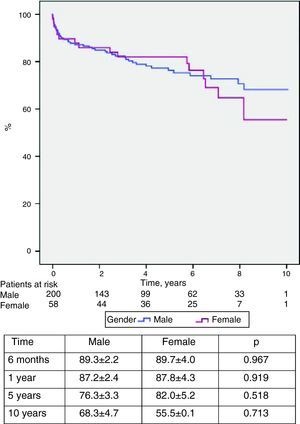
Mortality and survivalTwelve patients (4.7%) died in the first 30 days or during hospital stay, due to graft failure or stroke (five each) and hyperacute rejection (two), and 50 died during follow-up, giving an overall mortality of 24.0% (Table 6). The main cause of late death was infection (7.0%), followed by vascular (5.0%), neoplastic (3.9%) and cardiac complications (3.5%). Overall survival was 87.3±1.9% at one year, 77.7±2.8% at five years, 68.9±3.8% at eight years and 65.2±4.4% at 10 years (Figure 7). No difference was seen in survival between the sexes (Figure 8).
Cardiac transplantation is established as an effective treatment for end-stage HF that is refractory to other therapies. However, it is associated with a series of serious complications, some potentially fatal, which means it is essentially a palliative measure in the medium to long term. Ten-year survival is currently 50–60% and mean survival for those who survive the early post-transplant period is 12–13 years.1,3
The heart transplantation program of the University Hospital of Coimbra began in November 2003. Data on the first five years of activity were presented and analyzed in a report published in the Journal,9 which also presented a summary of the history and other aspects of cardiac transplantation in Portugal, including its place in the European context, and so this will not be addressed further here.
In the present study we analyze the results obtained in 258 patients transplanted in the first 10 years of the program. There were thus an average of 25 transplants per year, which fulfills the program's overall objective and makes it the largest accumulated experience of any Portuguese center. This high volume of activity is bound to have an impact on post-transplantation outcomes, particularly in cases of greater urgency and/or higher risk; the relationship between volume and quality in transplantation is well established.10–12 Furthermore, the experience acquired has allowed a widening of indications, the exploration of alternative techniques and the use of marginal donors, expanding the frontiers of organ acceptance and use.
There has been a progressive increase in both the mean age of recipients and the number of patients hospitalized for decompensated HF at the time of transplantation, requiring intensive drug therapy and in some cases mechanical circulatory and ventilatory support. The result is a growing number of recipients in poor clinical condition, which increases not only the urgency of the transplantation but also the risk of failure. This is further exacerbated by the scale of priorities for cardiac transplantation used in Portugal, initially implemented by the Portuguese Transplantation Organization, which was replaced by the Authority for Blood and Transplantation Services and then the Portuguese Blood and Transplantation Institute (Table 7), which is based on the candidate's clinical status on entering the waiting list, and thus means that more critical patients move ahead of those whose clinical condition allows them to wait for longer. However, this scale – and others like it in other countries – neither defines nor quantifies patients’ risk profile. The immediate results of transplantation are known to be highly sensitive to the quality of the donor organ and the candidate's clinical state.13,14 These are the main factors responsible for some unsatisfactory outcomes in the immediate post-transplant phase.15,16
Degrees of priority for cardiac transplantation in Portugal.
| Degree of priority | ||
| I | Emergent | Acute graft failure |
| II | Cardiogenic shock with ventricular assistance (CPB, ECMO, VAD) | |
| III | Cardiogenic shock with intra-aortic balloon pump | |
| IV | Cardiogenic shock and ventilatory support | |
| V | Urgent | Hospitalized, under inotropic support, with malignant arrhythmia or on hemodialysis |
| VI | Hospitalized in the ICU for treatment of CHF on at least two occasions in the previous 6 months | |
| VII | Non-urgent | |
CHF: chronic heart failure; CPB: cardiopulmonary bypass; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; VAD: ventricular assist device.
Patients with lower priority, on the other hand, benefit from their home environment and family support, as well as greater mobility, which improve their psychological and physical state and lead to a marked reduction in the incidence and severity of post-transplantation nosocomial infection. These factors undoubtedly reduce these patients’ risk.17 However, Lietz and Miller5 and Mokadam et al.18 have shown that 32–48% of UNOS status 2 candidates, equivalent to the Portuguese degree VI–VII, suffered clinical deterioration while on the waiting list (UNOS, the United Network of Organ Sharing, is a private organization that manages the US organ transplant system). At the time of transplantation, patients in this group may therefore be in poor clinical condition, with intermittent instability, recurrent decompensation, or chronic persistent symptoms frequently associated with high morbidity.2
Another high-risk group is diabetic patients. As would be expected among transplant candidates, over a quarter (27.9%) were diabetic at the time of transplantation, while 29 (11.27%) developed new-onset diabetes in the first year. The relationship between immunosuppression, especially with corticosteroids and tacrolimus, and worsening or development of new-onset diabetes is well known, and diabetes is one of the main risk factors for arteriosclerosis involving the coronary arteries as well as the systemic circulation.19 In a previously published work, we found no significant differences in morbidity or mortality between diabetic and non-diabetic transplantees up to five years after the procedure,20 but this appears to change in the longer term.21
Diabetes is also an important factor in the genesis and propagation of infections, which are now the leading cause of late morbidity and mortality following transplantation, rather than acute rejection, which nowadays is rarely fatal. In our series, 43 patients (16.7%) developed serious infections in the first year, mostly pneumonia, requiring hospitalization and intravenous antibiotic therapy. Infection was the main cause of late mortality, followed by vascular and neoplastic complications. This is likely due to excessive immunosuppressive therapy; some centers report excellent results with considerably less aggressive immunosuppression protocols than standard regimens.22 The protocol initially used in our center has recently been modified, but it is too soon to assess the effect of this change on the main complications of transplantation.
Commonly associated with chronic rejection is GVD, the etiology and natural history of which are little understood. GVD appears late, generally after five years, and results in progressive severe graft dysfunction, leading to total loss and death unless the patient is retransplanted, which rarely occurs.23 Unusually, most cases of GVD in our series were diagnosed within three years of transplantation. When diagnosed early, the evolution of GVD can be significantly slowed by changing the immunosuppression protocol, particularly by introducing the mTOR inhibitors sirolimus and everolimus.
In an attempt to increase the number of donors, age limits have been progressively raised. The use of older donors does not appear to be as important as might be expected; in another study based on our experience, we showed similar survival in recipients of hearts from older donors, even in young patients. Donor age, at least up to sixty years, thus appears not to be a limiting factor.24–27 It is, however, recommended to minimize ischemia time in such hearts,28 and we therefore prefer donors referred by institutions closer to our center.
Another way to increase the supply of donors is to use hearts with minor structural abnormalities that can be corrected. Twelve patients in our series received hearts with mild to moderate mitral disease, all of which were known before harvesting except in one case that was corrected before transplantation, while two patients underwent tricuspid annuloplasty. In addition, two patients underwent surgical revascularization of the anterior descending artery, one with the mammary artery used in a previous surgical revascularization. Some of these cases have been described in previous publications.29,30 All these patients are currently well, with no complications related to these procedures, except for one who died of stroke a few weeks after transplantation.
There was a female donor–male recipient gender mismatch in 17% of our cases. Such mismatches are often associated with worse outcomes,31 but in our series there were no significant differences in complications or mortality in cases of gender mismatch, and so it is not taken to be a reason for donor rejection so long as other selection criteria, such as morphological matching (weight and BMI) are met.
Survival was 87% at one year, 78% at five years and 65% at 10 years, which compares favorably with the results of other series, including the ISHLT registry of more than 100 000 cases,1 although the latter began much earlier. It is perhaps too early to draw conclusions, since the decline in survival curves appears to become steeper after the first 10 years due to late complications such as GVD and cancer, as well as progressive deterioration in renal function.
Worsening renal function caused by drug toxicity is common in cardiac transplant patients.32,33 Preoperative creatinine levels fell in the first month following transplantation, but then rose progressively up to the end of the first year, exceeding initial values. In most cases they remained relatively stable during the first five years, but thereafter many patients suffered significant renal deterioration in renal function. Three patients have undergone renal transplantation and two others are under renal replacement therapy while awaiting transplantation.
ConclusionsThe number of patients awaiting cardiac transplantation is growing, unlike the supply of donors. The situation is exacerbated by the increase in the number of candidates with worsening clinical status (degrees of priority II–V). This has led on one hand to a widening of the frontiers of organ donation and on the other to more careful and pragmatic candidate selection.
Transplantees generally derive great benefit from cardiac transplantation in terms of both quality of life and increased longevity. However, a considerable number suffer severe complications, some of them fatal, and so continuous monitoring and care are essential. This places a major burden on transplant teams, and requires a specific organizational structure. For this reason, and because outcomes are heavily dependent on level of experience, cardiac (and indeed other forms of) transplantation should be limited to centers with a reasonable level of activity, and should on no account be dispersed.
Demand in this area in Portugal is currently met to a reasonable extent, but the number of centers is probably excessive for the population served. We thus recommend a reappraisal of the situation, in order to ensure the quality of care for patients with end-stage heart failure that is refractory to conventional, conservative therapies. The current competition for donors does not serve the goal of appropriate distribution between patients on the waiting list in different transplantation centers.
The fall in donor numbers in recent years may change the relatively favorable situation which we have enjoyed in the 10 years since our transplantation program began. However, actions to raise awareness and reorganization of the ways that donors are identified and registered are already having an effect. In 2013, which may come to be seen as a turning-point in all areas of transplantation, 30 patients were transplanted in our center alone, a figure that had previously only been attained in 2005. Nevertheless, we still have a long way to go before reaching our maximum potential.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors acknowledge the crucial contribution to the results described above of the following staff at the Organ Donation and Transplant Coordination Office (GCCOT) of the University Hospital of Coimbra: Dr. Ana Maria Calvão da Silva (director), Nurse Maria João Henriques and Nurse António Manuel Dias Alves. This office is the most active in Portugal, thanks to their dedication and the quality of their work.
Please cite this article as: Prieto D, Correia P, Batista M, et al. Uma década de transplantação cardíaca em Coimbra. O valor da experiência. Rev Port Cardiol. 2014;33:671–681.