Heart failure (HF) is a complex clinical syndrome that is a significant burden in hospitalisations, morbidity, and mortality. Although a significant effort has been made to better understand its consequences and current barriers in its management, there are still several gaps to address. The present work aimed to identify the views of a multidisciplinary group of health care professionals on HF awareness and literacy, diagnosis, treatment and organization of care, identifying current challenges and providing insights into the future.
MethodsA steering committee was established, including members of the Heart Failure Study Group of the Portuguese Society of Cardiology (GEIC-SPC), the Heart Failure Study Group of the Portuguese Society of Internal Medicine (NEIC-SPMI) and the Cardiovascular Study Group (GEsDCard) of the Portuguese Association of General and Family Medicine (APMGF). This steering committee produced a 16-statement questionnaire regarding different HF domains that was answered to by a diversified group of 152 cardiologists, internists, general practitioners, and nurses with an interest or dedicated to HF using a five-level Likert scale. Full agreement was defined as ≥80% of level 5 (fully agree) responses.
ResultsGlobally, consensus was achieved in all but one of the 16 statements. Full agreement was registered in seven statements, namely 3 of 4 statements for patient education and HF awareness and 2 in 4 statements of both HF diagnosis and healthcare organization, with proportions of fully agree responses ranging from 82.9% to 96.7%. None of the HF treatment statements registered full agreement but 3 of 4 achieved ≥80% of level 4 (agree) responses.
ConclusionThis document aims to be a call-to-action to improve HF patients’ quality of life and prognosis, by promoting a change in HF care in Portugal.
A insuficiência cardíaca (IC) é uma síndrome clínica complexa que representa uma carga significativa em termos de hospitalizações, morbilidade e mortalidade. Apesar do esforço significativo para melhor compreender as suas consequências e as atuais barreiras na sua gestão, ainda existem várias lacunas a colmatar. O presente trabalho teve como objetivo identificar os pontos de vista de um grupo multidisciplinar de profissionais de saúde sobre sensibilização e educação, diagnóstico, tratamento e a organização dos cuidados na IC, identificando os desafios presentes e proporcionando perspetivas futuras.
MétodosFoi criada uma comissão coordenadora, que incluiu membros do Grupo de Estudo da Insuficiência Cardíaca da Sociedade Portuguesa de Cardiologia (GEIC-SPC), do Núcleo de Estudos de Insuficiência Cardíaca da Sociedade Portuguesa de Medicina Interna (NEIC-SPMI) e do Grupo de Estudo Cardiovascular (GEsDCard) da Associação Portuguesa de Medicina Geral e Familiar (APMGF). Esta comissão coordenadora elaborou um questionário contendo 16 afirmações relativas a diferentes domínios da IC que foi respondido por um grupo diversificado de 152 cardiologistas, internistas, especialistas de medicina geral e familiar e enfermeiros com interesse na IC, através da utilização de uma escala de Likert de 5 pontos. A concordância total foi definida como ≥80% de respostas de nível 5 (concordo totalmente).
ResultadosGlobalmente foi alcançada uma concordância simples dos respondentes em todas as 16 afirmações, exceto numa. A concordância total foi registada em sete afirmações, nomeadamente, 3 de 4 afirmações sobre educação do doente e consciencialização sobre IC e 2 em 4 afirmações sobre diagnóstico de IC e sobre organização de saúde, com percentagens de respostas de totalmente de acordo variando entre 82,9% a 96,7%. Nenhuma das afirmações sobre tratamento de IC registou concordância total, mas 3 de 4 atingiram ≥80% de respostas de nível 4 (concordo).
ConclusãoEste documento pretende ser um apelo à ação para melhorar a qualidade de vida e o prognóstico dos doentes com IC, através da promoção de uma mudança dos cuidados prestados aos doentes com IC em Portugal.
Heart failure (HF) is a complex clinical syndrome characterised by debilitating symptoms and signs, caused by multifactorial structural and/or functional abnormalities of the heart, resulting in elevated intracardiac pressures and/or inadequate cardiac output at rest or during exercise.1–3 It affects 1–2% of the general adult population in high-income countries4 and is the leading cause of hospitalisation in individuals above 65 years old.3,5 A significant proportion of patients with HF die within five years after diagnosis,6 making HF mortality higher than most of common cancers, both in men and women,7 which is contrary to common belief.
Beyond the negative impact of HF on patients’ quality of life, this syndrome is a major burden for patients and caregivers and has a significant economic impact due to the escalating costs of hospitalisations and to the substantial loss of patients’ and caregivers’ productivity.3,6,8,9
In Portugal, HF prevalence estimates date back to 1998, with a global estimation of 4.36% based on the EPICA study.5 This study showed a progressive prevalence increase with age, from 1.36% in the group between 25 and 49 years old to 16.14% in those over 80.5
Currently, to address knowledge gaps on HF epidemiology, characteristics and burden of disease, a new study is ongoing in Portugal. PORTHOS study10 aims to determine the prevalence of HF and its subtypes, the distribution of comorbidities among patients with HF, and patients’ health-related quality of life. The first study results are expected by the end of 2023.
Nevertheless, today we know that due to the population ageing and the increasing prevalence of cardiovascular risk factors such as obesity and diabetes, HF prevalence is expected to rise by 30% in 203511 and HF-associated deaths by 73% in 2036, further increasing HF economic burden, which is expected to grow by 28% in 2036.2
In 2002, Fonseca et al. suggested that HF was only satisfactorily managed in Portugal and should be a national priority.12 More recently, another consensus statement also highlighted the need to increase awareness and improve HF management in Portugal.13 In the past two decades, there has been significant innovation in the available therapies that triggered a transversal evolution in managing HF patients, namely in the HF disease awareness, diagnosis, treatment, and organization of healthcare. However, significant gaps in each of these areas have yet to be identified and acted upon for a more effective response.
ObjectivesTOGETHER-PT (conTemporary reflectiOns regarding heart failure manaGEmenT – How to ovERcome the PorTuguese barriers) intends to be a call-to-action document to improve HF care in Portugal. This is a project of the Heart Failure Study Group of the Portuguese Society of Cardiology (GEIC-SPC), the Heart Failure Study Group of the Portuguese Society of Internal Medicine (NEIC-SPMI) and the Cardiovascular Study Group (GEsDCard) of the Portuguese Association of General and Family Medicine (APMGF) that aims to identify the views of a multidisciplinary group of Portuguese cardiologists, internists, general practitioners and nurses, on HF different domains, such as awareness and literacy programs, early diagnosis implementation policies, guideline-oriented treatment implementation and organization of care.
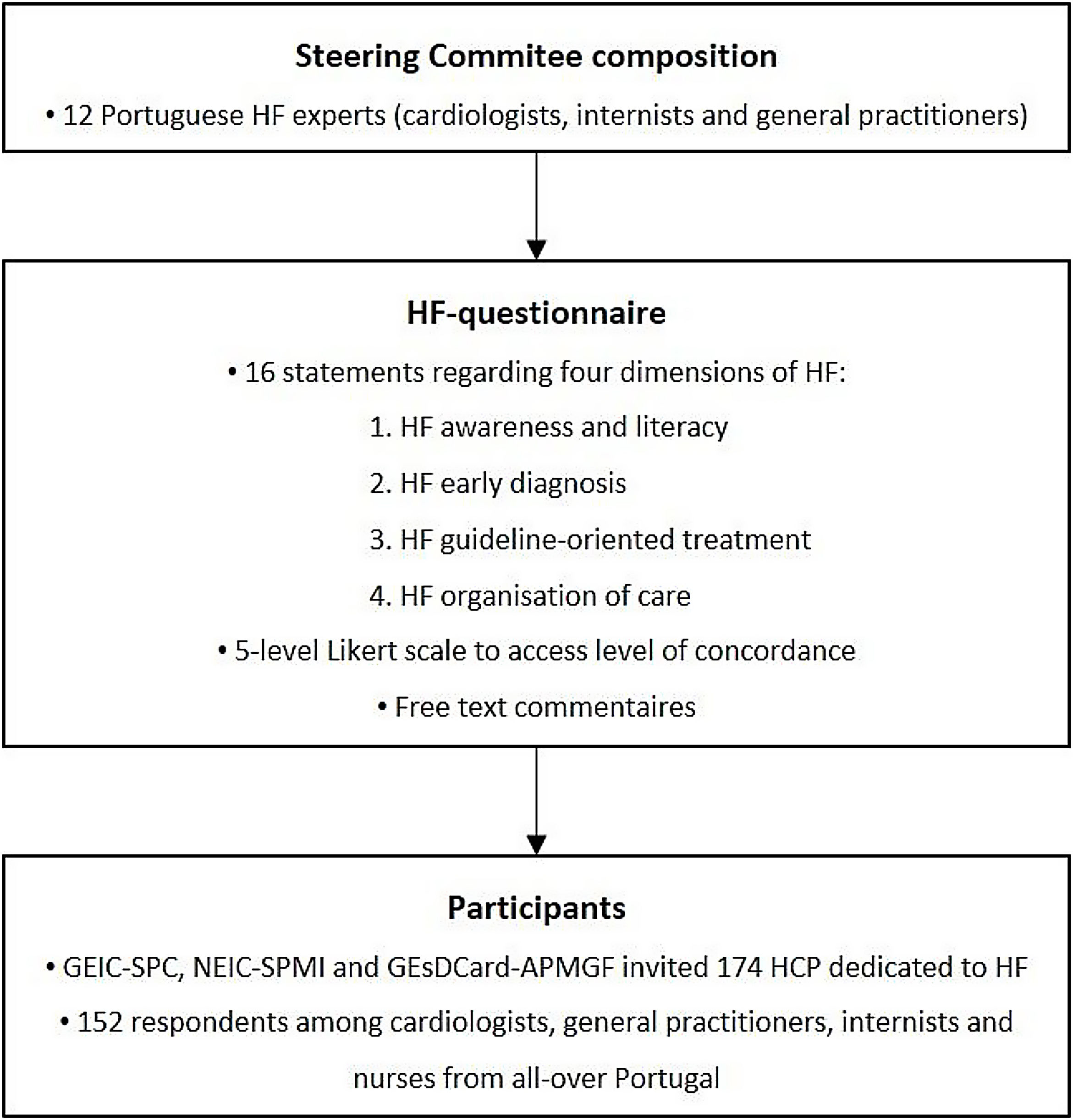
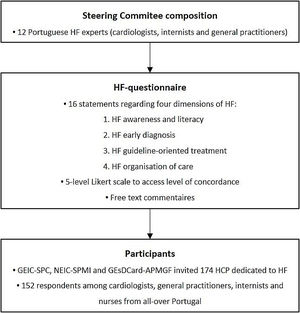
MethodsA panel of twelve Portuguese HF experts, including cardiologists, internists, and general practitioners, was invited to join the study Steering Committee. This Committee designed a questionnaire to collect the opinion of a group of HF experts on how to improve HF management in Portugal. The methodology used to build this questionnaire is summarised in Figure 1.
Questionnaire development method. GEIC-SPC: Heart Failure Study Group of the Portuguese Society of Cardiology; GEsDCard-APMGF: Cardiovascular Study Group of the Portuguese Association of General and Family Medicine; HCP: health care professionals; HF: heart failure; NEIC-SPMI: Heart Failure Study Group of the Portuguese Society of Internal Medicine.
The European Society of Cardiology (ESC) Guidelines on Heart Failure 20213 and other relevant contemporary literature were considered to be the current gold standard of HF management and they were taken into consideration to build the questionnaire. Four independent groups of the Steering Committee members were constituted. Each of these groups elaborated a set of four to six statements concerning each of the four dimensions of the TOGETHER-PT project:
- 1.
HF awareness and literacy.
- 2.
HF early diagnosis.
- 3.
HF guideline-oriented treatment.
- 4.
HF organization of care.
These statements were later analysed and voted on by all Study Steering Committee members, resulting in a final selection of 16 statements, four regarding each of the four dimensions mentioned above (Supplementary Table 1).
QuestionnaireA questionnaire was created with the selected 16 statements. It was used to quantify the level of agreement with each statement among the invited respondents. The questionnaire for physicians included the 16 statements regarding the four dimensions mentioned above. The questionnaire for nurses included the eight statements pertaining to two dimensions: HF awareness and literacy and HF care organization.
A five-level Likert scale was used for answering to each statement: level 1 – fully disagree, level 2 – disagree, level 3 – equipoise, level 4 – agree, and level 5 – fully agree. An additional free-text comment was allowed for each statement.
ParticipantsGEIC-SPC, NEIC-SPMI and GEsDCard-APMGF identified a convenience sample of 174 health care professionals (HCP). This group included cardiologists, general practitioners, internists, and nurses working at primary healthcare facilities, regional or central hospitals all over Portugal with interest, expertise, and experience in HF care. The 174 professionals were invited by e-mail and phone call to answer the questionnaire via an online form. After the first contact, two kind reminders by e-mail were sent.
Data analysisAgreement with each statement was classified as follows:
- •
“Full agreement” – when ≥80% of level 5 responses obtained;
- •
“Agreement” – when ≥80% of level 4 or 5 responses obtained;
- •
“No agreement” – when none of the above results was met.
- •
Free-text comments were qualitatively analysed.
This study involves the analysis of documents without direct or indirect intervention of participants. The prior review and approval by the competent Ethical Committee was waived. This study complied with the ethical principles for clinical research, specifically the confidentiality of respondents’ choices.
ResultsThe questionnaire was available online between 20 July and 20 September 2022.
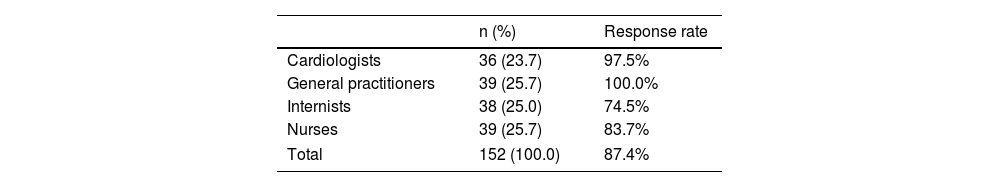
Among the 174 invited professionals, 152 (87.4% response rate) answered the questionnaire.
Response rate was balanced among the different professional groups (Table 1).
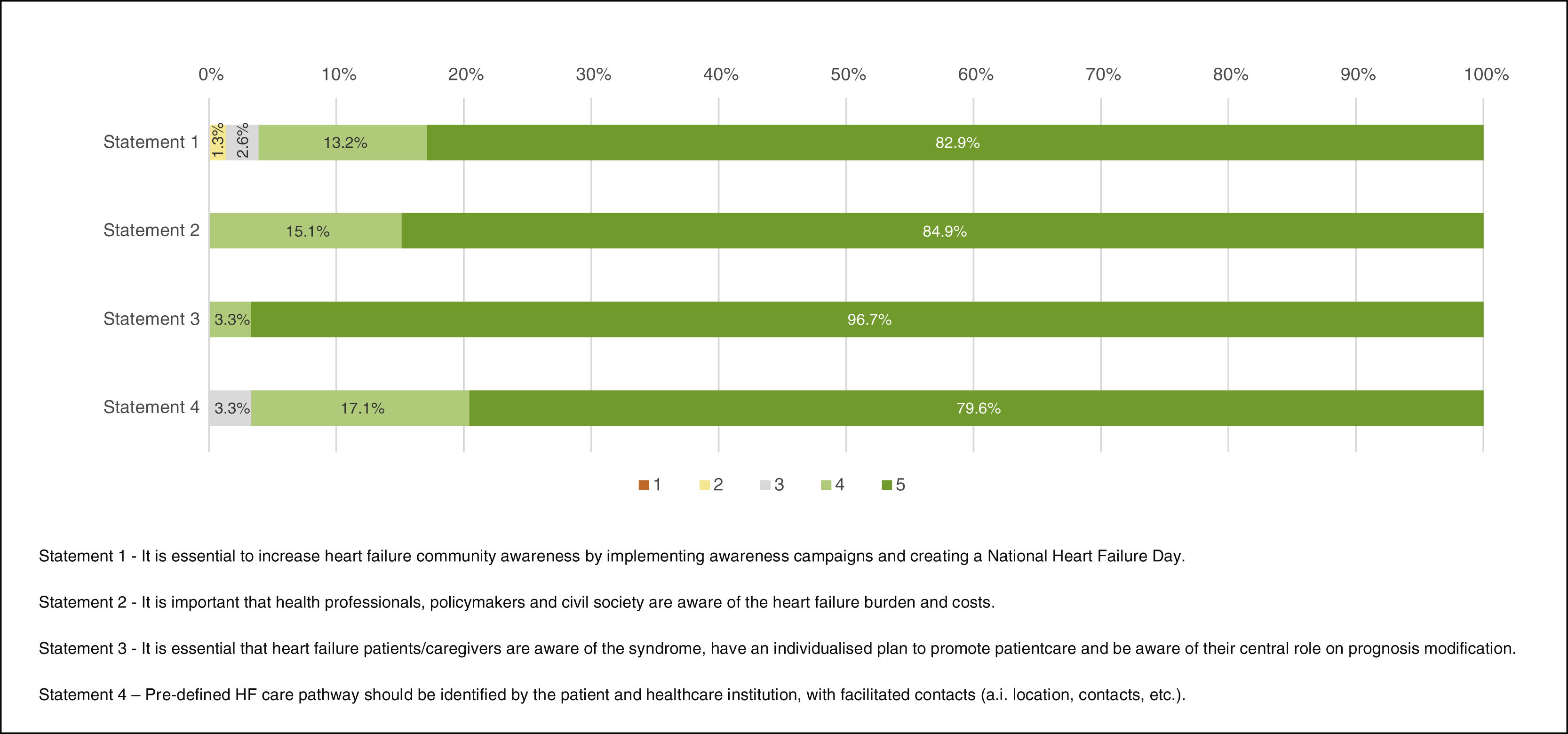
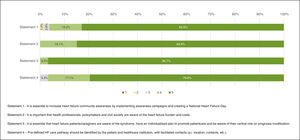
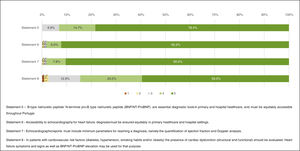
“Full agreement” was recorded for three of the four statements with concordance proportions of 82.9% (Statement 1 – “It is essential to increase heart failure community awareness by implementing awareness campaigns and creating a National Heart Failure Day.”), 84.9% (Statement 2 – “It is important that health professionals, policymakers and civil society are aware of the heart failure burden and costs.”) and 96.7% (Statement 3 – “It is essential that heart failure patients/caregivers are aware of the syndrome, have an individualised plan to promote patientcare and be aware of their central role on prognosis modification.”), and one statement obtained “Agreement”, with 96.7% of respondents answering 4 or 5 (Statement 4 – “Pre-defined HF care pathway should be identified by the patient and healthcare institution, with facilitated contacts (i.e. location, contacts, etc.).”) (Figure 2).
Participants strongly support national HF awareness campaigns among the general population and the health care community. They reinforced the utility of cardiovascular risk factors and HF preventive campaigns rather than the institution of a national day that would have little impact on population literacy. National HF awareness campaigns for the general public, and HF education campaigns conducted at primary healthcare facilities, nursing homes and schools, might also play an important role. Additionally, the development of HF awareness and education programs all year round was suggested. Respondents considered that an information campaign on HF's financial, social and disease burden should be developed targeting politicians and civil society. It was suggested that every healthcare institution should report their HF hospitalisations and/or HF outpatient care costs and establish targets to reduce them. This could be used to compare costs among different strategies and promote multidisciplinary care.
Experts provided suggestions on how to implement HF patient education: (1) to create a standard HF education package; (2) to use the “Heart failure zones”, a visual colour scale on HF signs and symptoms to encourage HF patients/caregivers to take action, namely, by contacting HF professionals; (3) to discuss with each patient/caregiver HF trajectory and prognosis; (4) to use a patient-centred approach in order to discuss HF treatment individual goals; and (5) to always involve caregivers and family physicians in HF patients’ education process.
Finally, participants highlighted the need to implement multidisciplinary teams at every level of care within the Portuguese National Health System. This would be essential to attend HF patients’ needs, thus overcoming the current absence of these programs in most hospitals and primary healthcare facilities. The relevance of nurse outpatient visits was underlined, as well as the need to implement digital health solutions to improve patient access to healthcare.
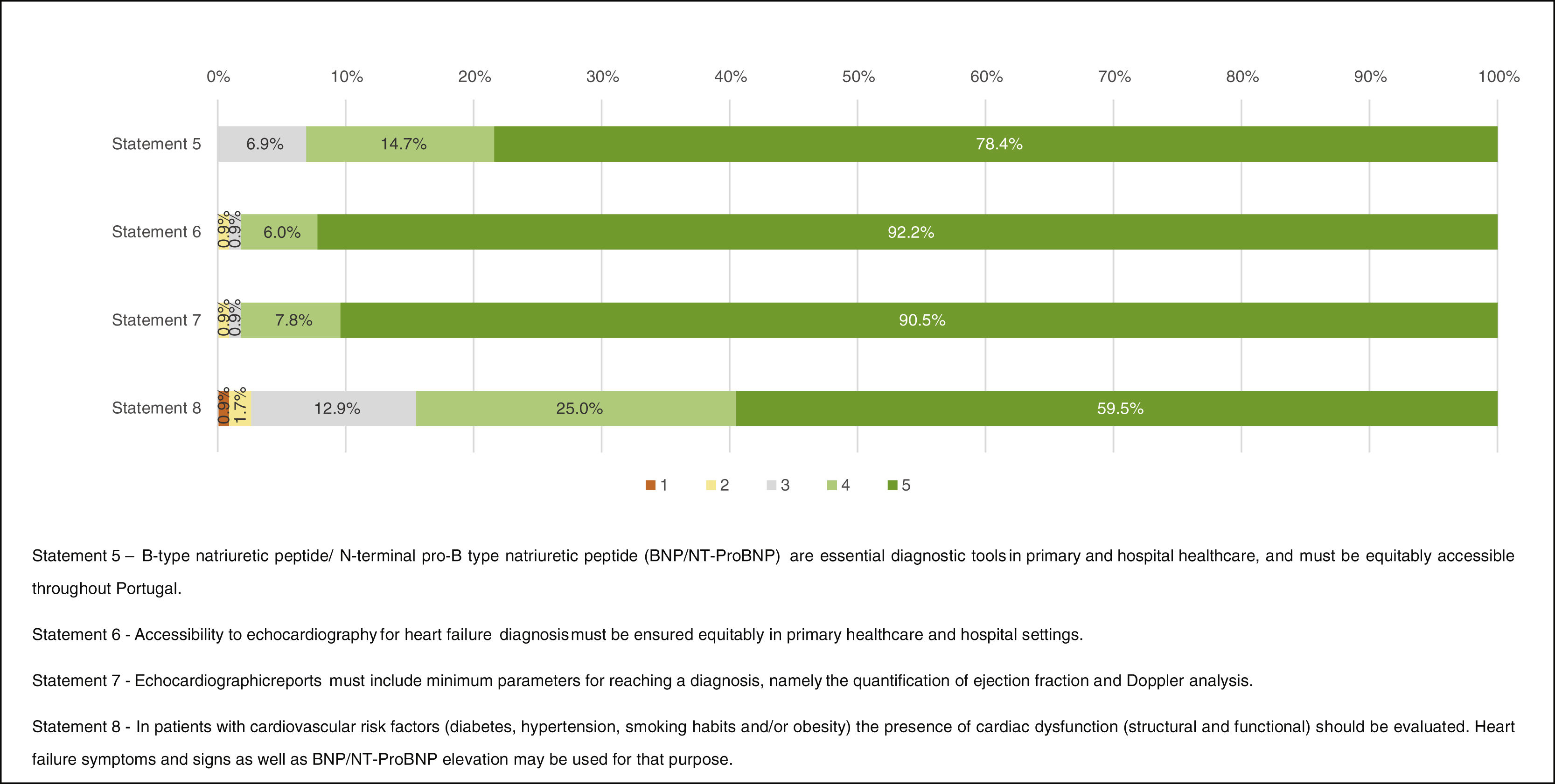
Heart failure diagnosis“Full agreement” was achieved regarding echocardiography (Statement 6 – “Accessibility to echocardiography for heart failure diagnosis must be ensured equitably in primary healthcare and hospital settings.”, 92.2% and Statement 7 – “Echocardiographic reports must include minimum parameters for reaching a diagnosis, namely the quantification of ejection fraction and Doppler analysis.”, 90.5%). “Agreement” was achieved regarding the role of natriuretic peptides (NPs) (Statement 5 – “B-type natriuretic peptide/N-terminal pro-B-type natriuretic peptide [BNP/NT-ProBNP] are essential diagnostic tools in primary and hospital healthcare, and must be equitably accessible throughout Portugal.”, 93.1% and Statement 8 – “In patients with cardiovascular risk factors (diabetes, hypertension, smoking habits and/or obesity) the presence of cardiac dysfunction (structural and functional) should be evaluated. Heart failure symptoms and signs as well as BNP/NT-ProBNP elevation may be used for that purpose.”, 84.5%) (Figure 3).
Respondents agreed on the relevance of echocardiography for establishing HF diagnosis. They also considered that access to good quality echocardiograms should be assured equitably both in outpatient and hospital settings. Essential echocardiographic parameters, such as ejection fraction and Doppler evaluation should always be included in the echocardiogram report. This was considered fundamental for a “comprehensive” and appropriate diagnosis.
Equitable access to NPs in the ambulatory and hospital setting at a national level was deemed crucial. NP testing reimbursement among primary care physicians, as well as education on its appropriate use, was considered necessary.
In asymptomatic patients at risk of developing HF (e.g., patients with diabetes, obesity or hypertension), the utility of NP gathered less level of agreement.
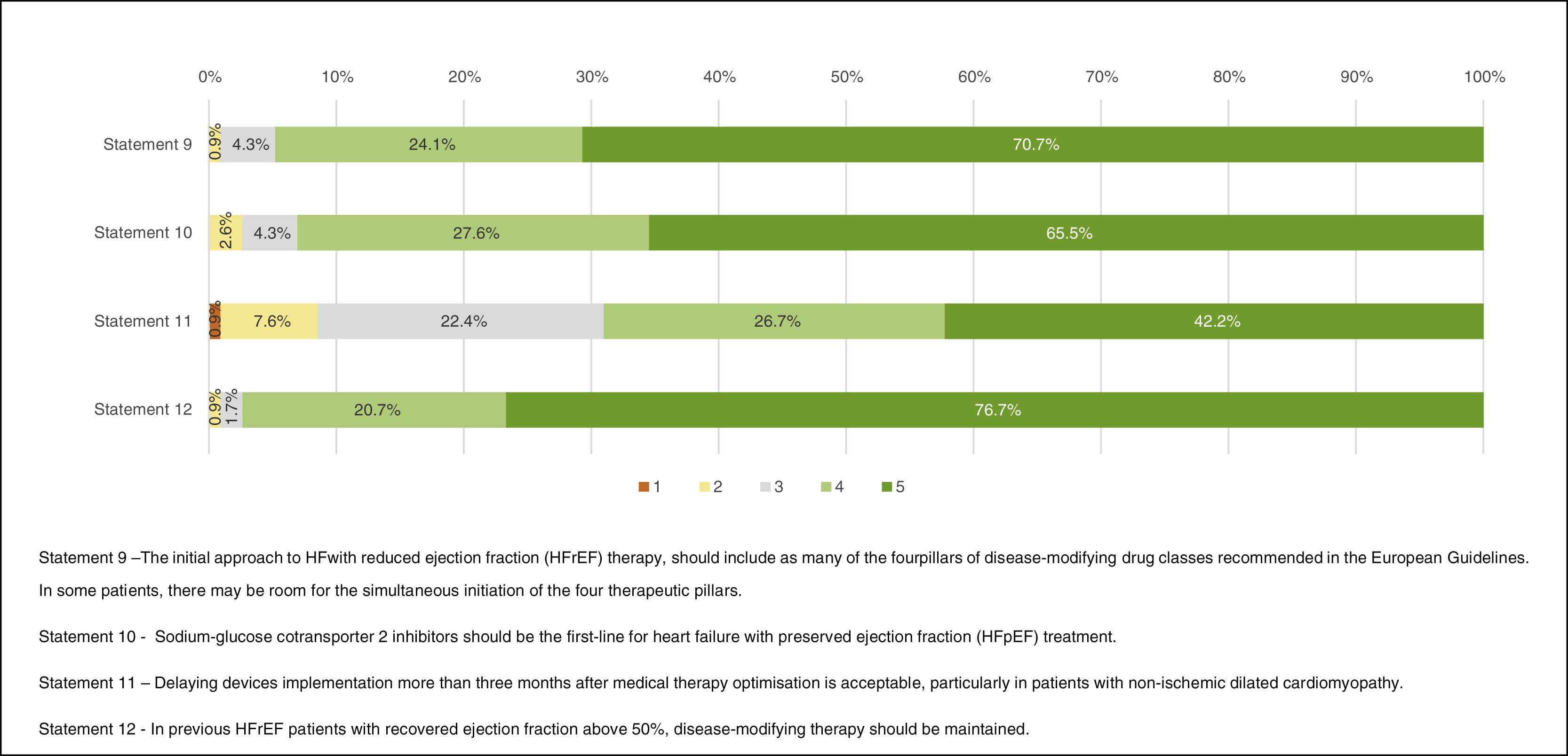
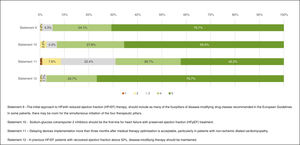
Heart failure treatment optionsResponses regarding treatment options are presented in Figure 4. “Agreement” was obtained in Statement 9 – “The initial approach to HF with reduced ejection fraction (HFrEF) therapy, should include as many of the four pillars of disease-modifying drug classes recommended in the European Guidelines. In some patients, there may be room for the simultaneous initiation of the four therapeutic pillars.” (94.8%), 10 – “Sodium-glucose cotransporter 2 inhibitors should be the first-line for heart failure with preserved ejection fraction (HFpEF) treatment.” (93.1%) and 12 – “In previous HFrEF patients with recovered ejection fraction above 50%, disease-modifying therapy should be maintained.” (97.4%).
The fundamental relevance of implementing the four pillars of disease-modifying therapy in all HF with reduced ejection fraction patients was highlighted. Drug initiation and dose titration should be done as soon as possible. However, in some patients, simultaneous initiation of all the above drugs may not be possible. The occasional occurrence of drug intolerance during up-titration may be a barrier in some cases. This is also true concerning drug costs in those economically fragile.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i; dapagliflozin and empagliflozin) were accepted as the first-line treatment for HFpEF.
Participants agreed that disease-modifying treatment should be maintained in HF patients with improved left ventricular ejection fraction (LVEF). Improved LVEF is defined by an increase ≥10-point from baseline in patients with previous LVEF ≤40%. A second measurement of LVEF >40% is necessary for diagnosis. In cases of LVEF improvement, when in doubt of a reversible cause, most experts would agree to maintain disease-modifying therapy.
The 11th Statement (“Delaying devices implementation more than three months after medical therapy optimisation is acceptable, particularly in patients with non-ischemic dilated cardiomyopathy.”) did not gather “Agreement”. Commentary suggested that this issue is not to be approached by non-cardiologist physicians.
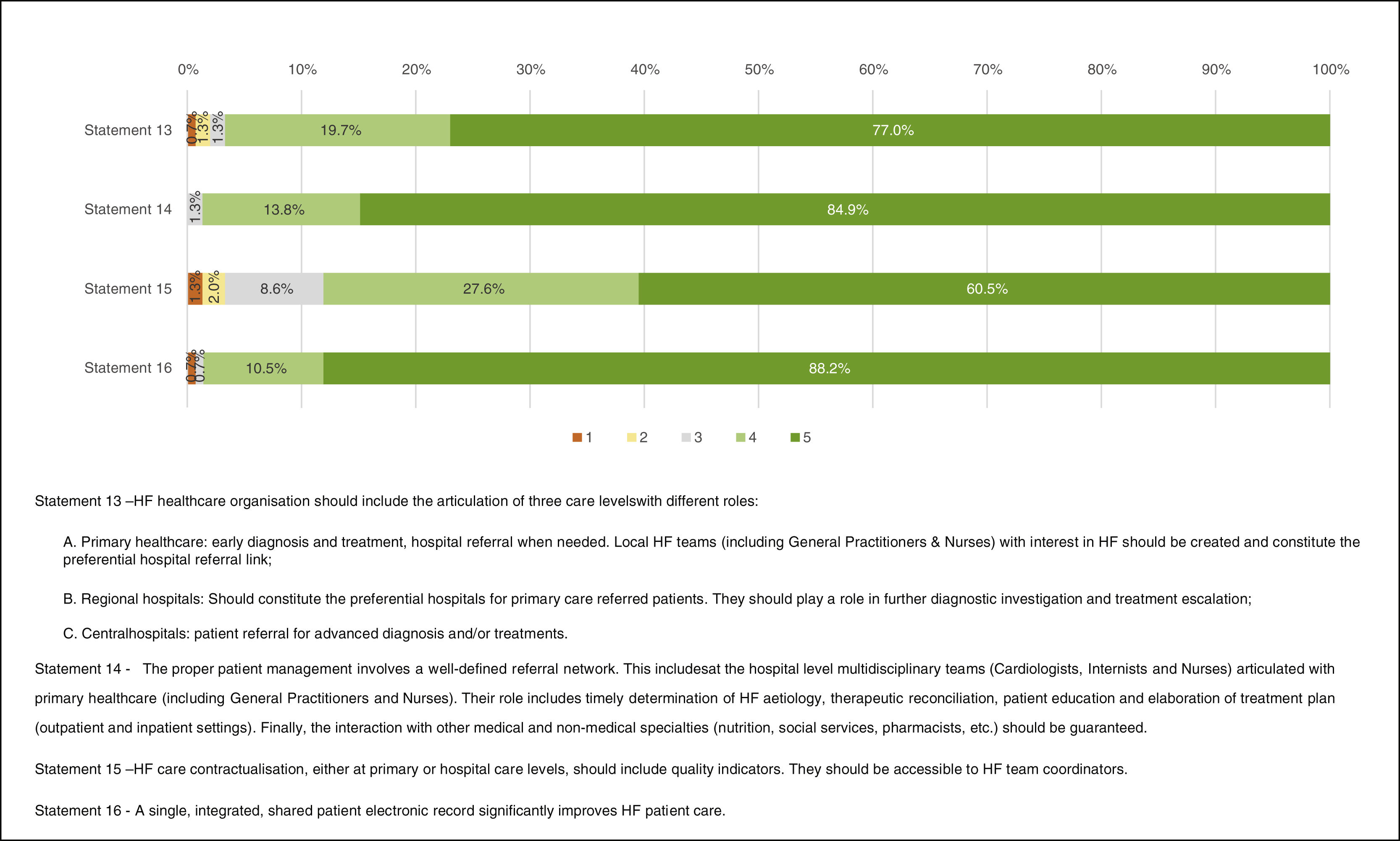
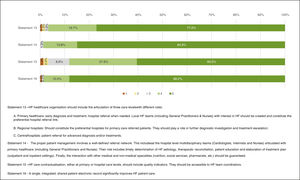
Healthcare organizationFigure 5 presents the results regarding healthcare organization. “Full agreement” was obtained in Statement 14 – “The proper patient management involves a well-defined referral network. This includes in hospital level multidisciplinary teams (cardiologists, internists, and nurses) engaged with primary healthcare (including general practitioners and nurses). Their role includes timely determination of HF etiology, therapeutic reconciliation, patient education and elaboration of treatment plan (outpatient and inpatient settings). Finally, the interaction with other medical and non-medical specialties (nutrition, social services, pharmacists, etc.) should be guaranteed.” (84.9%) and 16 – “A single, integrated, shared patient electronic record significantly improves HF patient care.” (88.2%). “Agreement” was obtained in Statements 13 – “HF healthcare organization should include the articulation of three care levels with different roles: A. Primary healthcare; B. Regional hospitals; C. Central hospitals.” (96.7%) and 15 – “HF care contractualisation, either at primary or hospital care levels, should include quality indicators. They should be accessible to HF team coordinators.” (88.1%).
A well-defined integrated healthcare system including the various HF care levels and using shared electronic medical records and information was considered crucial. Critical points in providing appropriate care to HF patients included the establishment of multidisciplinary integrated teams and the creation of easy communication pathways. Health professionals’ education was highlighted as being fundamental. Cardiac rehabilitation is also a central part of HF care. Finally, the contribution of psychologists was referred as of most importance.
Although contractualisation was accepted, some respondents questioned HF quality indicators utility in clinical practice. They argued that it does not ensure care improvement, stressing the need for defining the correct quality of care indicators to successfully implement contractualisation.
DiscussionPatient education and awareness in heart failureThe respondents (HCPs) identified HF literacy as an important unmet need among Portuguese HF patients. This is also true when considering the general population and political decision-makers. Indeed, community awareness of HF is low, and strategies to educate the public are needed.13,14 In a 2005 survey conducted in nine European countries, 86% of respondents affirmed having already heard of HF; however, only 3% could correctly identify HF from a description of typical symptoms and signs. Additionally, 34% believed HF was a normal consequence of ageing, and 67% thought that HF patients live longer than cancer patients.14
The 2021 European Society of Cardiolgy (ESC) Guidelines recommend self-management strategies to reduce the risk of HF hospitalization and mortality.3 Patient education is particularly important to achieve this. The TOGETHER-PT participants recognized the need to promote patient/caregiver education and empowerment. ESC recommends the use of online platforms (e.g., HF matters) to promote HF patient self-education.3 In Portugal, patient associations and scientific societies promote HF patient education and support.
Policymakers should designate HF as a health policy and management priority.13 HF should be classified as a chronic disabling status, like other diseases such as diabetes or cancer. This would ensure simplified access to healthcare facilities and allow patient care optimization with a potential impact on HF hospitalizations and quality of life.
Heart failure diagnosisAccording to the ESC Guidelines, HF diagnosis is based on typical symptoms and signs, in addition to the information provided by ECG, NP and echocardiography.3
The lack of access to complementary diagnostic tests, such as NP and echocardiography at the primary healthcare level, has been described in Portugal.13,15 This is particularly relevant since registry data showed that, although 40% of patients present symptoms that should result in an earlier diagnosis at the primary healthcare level, 80% of HF diagnoses are made at the hospital16.
The participants reinforced the central role of NPs, equitably accessed, as a diagnosis tool, if proper training is given to ensure their adequate use and interpretation. However, as previously stated, in Portugal, BNP/NT-ProBNP measurements are not reimbursed in primary healthcare. This situation should be revised as their use enables the optimization of echocardiography use, resulting in cost-reduction for the Portuguese public health system.13,17,18
The participants agreed with the relevance of the availability of high-quality echocardiograms, including Doppler assessment. They are central to HF diagnosis and treatment prioritization and should be equitably accessible. Echocardiograms should have a standardized report and be performed timely, in accordance with the literature, such as the Portuguese Society of Cardiology recommendations.13,19,20
Patients at risk of developing HF (patients with cardiovascular risk factors, such as diabetes, hypertension, smoking habits and/or obesity) should be screened early by actively looking for HF signs and symptoms. There is no specific mention to dyslipidemia because abnormal lipid concentrations, by themselves, are recognized as an important independent risk factor for CV diseases, but mainly for atherosclerotic events. On the other hand, the use of NP for screening was not consensual among the consulted panel of health professionals. In the recently published HF classification update,21 subjects without signs or symptoms of HF, without evidence of structural heart disease or abnormal cardiac function but with elevated NPs or cardiac troponin levels could be classified as pre-HF patients, formerly stage B. In the primary healthcare setting, in the non-acute setting, the diagnostic value of NPs, in addition to the presence of signs and symptoms of HF, relies on their high negative predictive value, helping physicians to rule out HF.3
Heart failure treatmentThe main goals of HF treatment include the control of symptoms and signs, improvement of functional capacity and quality of life, and decrease of hospitalizations and mortality.3 In recent years new therapies emerged with unprecedented results that contributed to these goals.22–29
The PARADIGM-HF,23 DAPA-HF24 and EMPEROR-REDUCED studies25 led to the updated HFrEF treatment algorithm of the 2021 ESC HF Guidelines. It proposes a four-pillar strategy involving the association of an angiotensin-converting enzyme inhibitor (ACEi), an angiotensin receptor-neprilysin inhibitor (ARNI), an angiotensin II receptor blocker (ARB), a beta-blocker (BB), a mineralocorticoid receptor antagonist (MRA) and an SGLT2i.3
These four pillars of therapy are recognized as first-line HFrEF treatment by the Portuguese health care professionals gathered in the TOGETHER-PT project.
This is particularly important because, according to registry data, guideline-directed medical therapies (GDMT) are not being implemented consistently in clinical practice.30–33 The CHAMP-HF registry30 showed that the use of GDMT was under 75% in stable outpatients with HFrEF, both regarding the prescription of disease-modifying drugs and the achievement of target dosage. Only 1% of patients were treated simultaneously with target doses of ACEi/ARB/ARNI, BB, and MRA therapy, and less than 25% simultaneously received any dose of all three medications.30 Additionally, data from the ESC Long-Term Registry32 regarding patients with chronic HFrEF showed that, with respect to the target dosages of GDMT, far fewer than one-third of the patients were on the target dosages suggested by the current Guidelines: 29.3% for ACEi, 24.1% for ARB,17.5% for BB, and 30.5% for MRA. Data from ESC HFA EORP 2011–201631 showed GDMT use rates of 85.7% for ACEi/ARB/, 88.7% for BB and 58.8% for MRAs. Additionally, the EVOLUTION-HF observational study34 showed that the initiation of dapagliflozin and sacubitril/valsartan was delayed compared with other GDMT. In this study, only a few patients were on target doses. The above registries reveal the unmet need of initiating treatment early with ensuing rapid dose up-titration in order to improve HFrEF patients’ prognosis.34
In TOGETHER-PT, the participants considered SGLT2i as the first-line option for HFpEF management.
SGLT2i are the only treatment reducing morbidity and mortality in HFpEF.22,26,27 The 2022 AHA/ACC/HFSA Guidelines for the management of HF35 give a class II-A recommendation for using SGLT2i in HFpEF to reduce mortality and HF hospitalizations, based mainly on EMPEROR-preserved trial results.26,27,36 DELIVER22 trial, published in 2022, after the release of the 2022 AHA/ACC/HFSA Guidelines, reinforced the evidence of SGLT2i for the reduction of CV death and worsening of HF in subacute and chronic outpatient setting with LVEF >40%. Additionally, a pre-specified pooled analysis of DAPA-HF and DELIVER29 showed a significant CV death reduction across the HF spectrum. All these compelling data on SGLT2i reinforces their role on HF patients regardless of LVEF.
Participants also globally agreed on the importance of maintaining GDMT in HF patients with improved ejection fraction. This is particularly important because, following resolution of symptoms and recovery in cardiac function, patients frequently ask to stop medications. According to ESC Guidelines,3 these patients should continue on their medication, as it is challenging to be certain of a complete and sustained recovery. This is in accordance with the TRED-HF trial37 that showed that withdrawal from treatment was associated with relapse. In addition, the DELIVER trial38 included HF patients with improved ejection fraction. Of 6263 patients, 18% had improved ejection fraction, and their benefit with dapagliflozin regarding both primary and secondary endpoints was consistent with the overall population.38 This data suggest that patients with improved ejection fraction should continue treatment with disease-modifying therapies and initiate dapagliflozin if it was not initiated before.
Finally, despite Statement 11 (“Delaying devices implementation more than three months after medical therapy optimization is acceptable, particularly in patients with non-ischemic dilated cardiomyopathy.”) not scoring enough for “Agreement”, we acknowledge significant limitations to interpreting the results. In a closer analysis of the results according to medical specialty, there was an agreement of 89.7% if we consider only the answers from the cardiologists. Although this analysis was not previously specified, it is particularly relevant since the device implementation is usually a decision of cardiologists and/or the heart team. Thus, we believe the inclusion of general practitioners significantly biased the results.
Healthcare organizationA high level of agreement was observed regarding the healthcare organization statements. Strategies to coordinate the different healthcare levels, develop optimized care and effective prevention programs are needed to decrease the economic and social impact of HF.3
The recent ESC, the National Institute for Health and Care Excellence and AHA/ACC/HFSA Guidelines recommend multidisciplinary HF management programs (HF-MPs).3,35,39 Multidisciplinary teams (MDTs) are decisive in providing care to HF patients along their vital journey, including onset, hospital admissions and outpatient journey during apparent stability. Medical, devices and surgical therapies should be provided as well as rehabilitation, advanced, palliative and terminal care.3 The MDT should be led by an HF specialist, usually a cardiologist or internal medicine specialist, and include nurses with HF training, who are central players in these teams.40 Care needs should be coordinated carefully with the general practitioners, and permanent communication is essential to succeed.40,41 Pharmacists, physiotherapists, nutritionists, palliative care teams, psychologists, social workers, occupational therapists, and administrators should also integrate the multidisciplinary teams providing holistic and patient-centric care to HF patients.40
Several implemented HF-MPs have shown positive results in HF readmissions. A recent meta-analysis including 38 randomized clinical trials (RCTs), concluded that multidisciplinary interventions significantly reduced readmissions up to six months post the index HF hospitalisation.42 A previous network meta-analysis including 53 RCTs showed a reduction of all-cause mortality after hospitalization for HF due to disease-management clinics and home visits by nurses compared to usual care.43 In Spain, the STOP-HF-Clinic intervention resulted in a 50% significant reduction in all-cause 30-day readmission, mainly driven by reduced HF-related readmissions.44
Models of care may vary in their components and should be adapted to healthcare systems, local conditions and patients’ needs.
Strengths and limitationsThis project has several strengths that need to be emphasized. To our knowledge, TOGETHER-PT is the largest HF questionnaire ever conducted in Portugal, including the participation of 152 HCPs highly interested in HF. This work is a glimpse into the views of Portuguese physicians and nurses on the issues related to HF awareness, diagnosis, treatment, and organization of care. TOGETHER-PT questionnaire included a sizeable number of participants not only from different medical specialties but also nurses.
Finally, TOGETHER-PT was designed by a joint effort of the three main national study groups related to HF – GEIC-SPC, NEIC-SPMI and GEsdCard-APMGF.
There are some limitations to this study. The first is the selection bias. The HCP invited into TOGETHER-PT were selected by GEIC-SPC, NEIC-SPMI and GEsdCard-APMGF. The authors cannot rule out that this bias influenced the results. However, the primary goal was not to have a representative sample of the Portuguese HF treating community. In contrast, we wanted to obtain the opinion of a sample of the most recognizable Portuguese HF experts. We believe the sample of 152 participants from diversified geographies and care contexts confers a varied perspective on Portuguese HF-related issues.
Thus, we consider that TOGETHER-PT can contribute to the improvement of HF care in Portugal.
ConclusionsTOGETHER-PT is the largest project conducted in Portugal aiming to obtain the perspectives of a broad group of HF experts on HF awareness, diagnosis, treatment, and organization of care in Portugal. There was significant consensus expressed by the participants regarding the four HF dimensions addressed.
This project, endorsed by GEIC, NEIC and GEsdCard, aims at being a promoter of change on HF landscape in Portugal to improve Portuguese HF patients’ quality of life and prognosis.
This joint effort may inspire further cooperative actions among scientific societies and other relevant stakeholders to promote change in this field in our country.
FundingFinancial support for the preparation of this article was provided by AstraZeneca. AstraZeneca had no role in the writing of the report and in the decision to submit the article for publication.
Conflict of interestAurora Andrade has received speaker or advisory boards fees from Novartis, AstraZeneca, Servier, Orion, Boehringer Ingelheim and Bayer.
Brenda Moura has received advisory and speaker fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Servier, Novartis, and Vifor Pharma.
Inês Araújo has received speaker or advisory board fees from AstraZeneca, Bayer, Bial, OrionPharma, Novartis, Servier, ViforPharma.
Irene Marques has received consultancy or speaker fees from AstraZeneca, Bayer, Bial, Daiichi Sankyo, Novartis Farma, Pfizer, Roche Diagnostics, Servier, CSL Vifor.
Joana Pimenta has received speaker or advisory board fees from AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Lilly, Merck, MSD, Novartis, Pfizer, Roche Diagnostics, Servier Portugal, Vifor Pharma.
Jonathan dos Santos has received fees from Viatris, Boehringer Ingelheim, Lilly, Novartis, Pfizer and Servier.
João Agostinho has received speaker and advisory board participation fees from AstraZeneca, Bayer, Bial, Boehringer Ingelheim and Lilly, Daiichi Sankyo, Ferrer, Menarini, Novartis, Orion Pharma, Pfizer, Servier and Vifor Pharma.
João Ferreira has received consulting fees from AstraZeneca, Novartis, Boehringer Ingelheim, Bayer and Jansen outside this work.
José Silva-Cardoso has received speaker and consultant fees, advisory board participation fees, or investigational grants from Abbott, AstraZeneca Pharmaceuticals, Bial, Boehringer Ingelheim, Menarini, Merck Serono, Merck Sharp & Dohme, Novartis, Orion, Pfizer, Sanofi, Servier, and Vifor Pharma.
Paulo Santos has received fees from Bial, AstraZeneca, Lilly, Tecnimede, Boehringer Ingelheim, MSD, Laboratórios Victória and Medinfar.
Marisa Peres and Pedro Morais Sarmento have no declarations of interest.
We acknowledge all the cardiologists, general practitioners, internists, and nurses who took some time to answer the questionnaire and to contribute with valuable inputs for the thematic discussion here presented (Supplementary Table 2).
The authors acknowledge writing assistance provided by Ana Santos from Prime Focus, funded by AstraZeneca.