Despite improvements in treatment, mortality associated with cardiogenic shock (CS) following acute myocardial infarction remains high.
AimTo compare two groups of patients admitted with CS over a 10-year time span.
MethodsWe performed a retrospective analysis of two patient populations presenting with CS admitted in the periods May 1998-May 2001 (group A) and May 2008-May 2011 (group B). Clinical characteristics, diagnostic methods, treatment and outcomes were compared, and independent predictors of death at six months were analyzed.
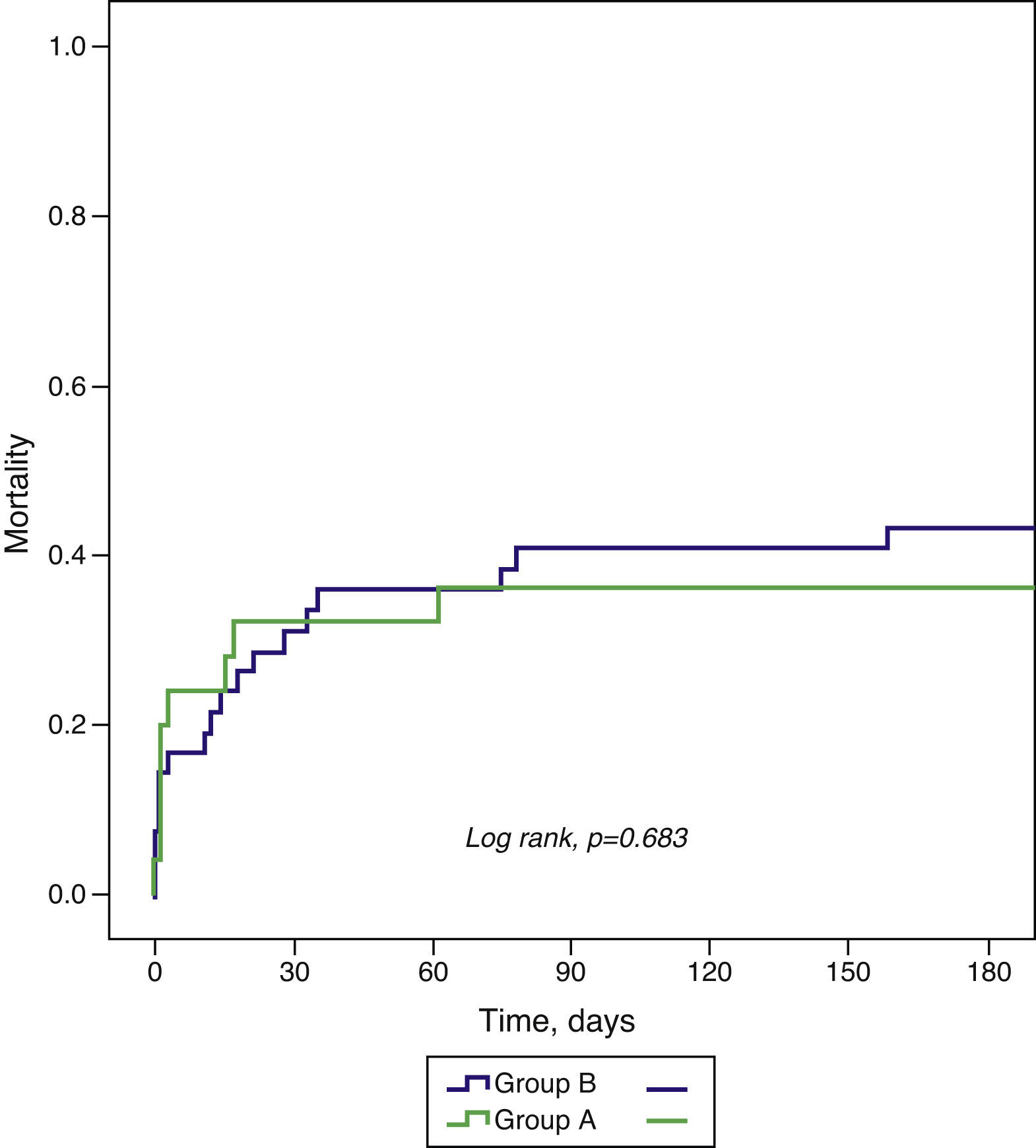
ResultsThe incidence of CS was 3.7% in group A (n=25) and 4.8% in group B (n=42). There were no significant differences in clinical characteristics except for age (60.2±12.3 vs. 66.5±11.3 years; p=0.043) and the proportion of patients admitted within six hours of symptom onset (29.2% vs. 54.8%, p=0.045). There was a reduction in use of pulmonary artery catheterization (52.0% vs. 19.0%, p=0.005) but an increase in dialysis (4.0% vs. 28.6%, p=0.014). There was no difference in the proportion of patients reperfused within 12 hours or revascularized, but use of percutaneous coronary intervention (PCI) increased (75.0% vs. 92.9%, p=0.042). There were no differences in outcomes, including mortality at 30 days (32.0 vs. 35.7%; p=0.757) and six months (36.0 vs. 42.9%; p=0.683). Diabetes was the sole baseline characteristic identified as an independent predictor of death at six months (hazard ratio [HR] 3.02; 95% confidence interval [CI] 1.38-6.60; p=0.006) and mortality was lower among revascularized patients (HR 0.11; 95% CI 0.03-0.42; p=0.001).
ConclusionsOver a 10-year time span, despite earlier hospital admission and increased use of support therapies and PCI, short- and medium-term mortality remained unchanged.
Apesar dos avanços terapêuticos, a letalidade do choque cardiogénico (CC) associado ao enfarte agudo do miocárdio (EAM) permanece elevada.
ObjetivoComparar 2 grupos de doentes com CC associado ao EAM, admitidos com um intervalo de 10 anos.
MétodosAnálise retrospetiva de 2 populações de doentes com CC associado ao EAM admitidos entre maio/1998-maio/2001 (Grupo A) e maio/2008-maio/2011 (Grupo B). Compararam-se as características clínicas, diagnóstico, tratamento e complicações e analisaram-se os preditores de morte aos 6 meses.
ResultadosA incidência de CC foi 3,7% no Grupo A (n = 25) e 4,8% no Grupo B (n = 42). Não existiram diferenças significativas nas características demográficas e clínicas, exceto na idade (60,2 ± 12,3 versus 66,5 ± 11,3 anos; p = 0,043) e doentes admitidos com < 6 h de sintomas (29,2 versus 54,8%, p = 0,045). O cateterismo da artéria pulmonar diminuiu (52,0 versus 19,0%, p = 0,005) e as técnicas dialíticas aumentaram (4,0 versus 28,6%, p = 0,014). A proporção de doentes reperfundidos nas primeiras 12 h ou revascularizados foi semelhante, mas a intervenção coronária percutânea (ICP) aumentou (75,0 versus 92,9%, p = 0,042). As complicações intra-hospitalares, mortalidade aos 30 d (32,0 versus 35,7%; p = 0,757) e 6 meses (36,0 versus 42,9%; p = 0,683) não diferiram. A diabetes foi a única característica basal preditora independente de morte aos 6 meses (HR 3,02; IC 95% 1,38-6,60; p = 0,006) e os doentes revascularizados apresentaram menor mortalidade (HR 0,11; IC95% 0,03-0,42; p = 0,001).
ConclusãoNos últimos 10 anos, apesar da chegada mais precoce dos doentes ao hospital, da maior utilização de algumas medidas de suporte e acesso à ICP, a mortalidade a curto e médio prazo não se alterou.
Cardiogenic shock (CS) due to left ventricular pump failure remains a major challenge in cardiology, and is the main cause of in-hospital death following myocardial infarction (MI).1,2 The incidence of CS associated with ST-segment MI is 5%-8%, a third of cases being diagnosed at hospital admission.1,2
Left ventricular pump failure, generally the result of anterior MI, triggers various mechanisms involved in the genesis and perpetuation of CS. Of these, neurohumoral cascade activation plays a central role, leading to the release of pro-inflammatory mediators such as cytokines and nitric oxide (NO) that are involved in the systemic inflammatory response seen in CS, resulting in systemic hypoperfusion and multiple organ failure.3,4 A high level of clinical suspicion, based mainly on signs of hemodynamic instability, is crucial to appropriate management of these patients.
The only treatment that has been shown to reduce mortality in MI complicated by CS is early coronary revascularization,5 which should not be delayed, irrespective of whether thrombolysis has been performed.6 The number of patients undergoing percutaneous coronary intervention (PCI) in the context of CS has progressively increased in recent years.2 Unlike MI patients who are not hemodynamically unstable, in whom only the culprit lesion should be treated, complete revascularization of all critical coronary lesions is a class I recommendation, level of evidence B, for patients with CS in the European Society of Cardiology guidelines on myocardial revascularization,7 which reflects the fact that two or more coronary territories are frequently involved in the genesis of CS due to pump failure.8
Besides revascularization, other diagnostic and therapeutic measures are also crucial to management of CS, particularly vasopressor and inotropic agents, invasive mechanical ventilation, renal replacement therapy and mechanical support therapies such as intra-aortic balloon pump counterpulsation.4 When necessary, some of these measures can be begun in a pre-hospital context. Invasive hemodynamic monitoring, insertion of an arterial line and pulmonary artery catheterization to measure right and left pressures and outputs and calculate pulmonary and systemic vascular resistance are essential in assessing the efficacy of such measures.
Despite advances in the management and treatment of patients with CS, mortality has not changed significantly in recent years and remains high: around 50% of MI patients who suffer CS die in hospital.2 On the other hand, those who recover have good long-term survival, which makes it imperative to develop strategies to stabilize these patients, especially in the first hours following the event.
The aim of this study was to analyze changes in the clinical characteristics, treatment and mortality of patients with MI complicated by CS due to left ventricular pump failure over a 10-year time span.
MethodsPopulationWe performed a retrospective analysis of two patient populations admitted to the cardiac intensive care unit (CICU) with a diagnosis of CS following MI, in the periods May 1998-May 2001 (group A) and May 2008-May 2011 (group B).
Patients who developed CS during hospitalization were excluded from the analysis, as were those with CS due to mechanical complications or right ventricular failure following MI as assessed by transthoracic echocardiography. Patients were selected on the basis of data recorded prospectively using Cardiobase® software (Infortucano, Lisbon).
The diagnosis of CS at hospital admission was based on the following clinical criteria: persistent hypotension (systolic blood pressure [SBP] <90 mmHg) following intravenous rehydration therapy or need for vasopressor support to maintain SBP ≥90 mmHg, together with signs of peripheral hypoperfusion (oliguria or anuria or cold clammy extremities).5 For both periods under consideration, management included immediate coronary angiography with a view to revascularization. In most patients, insertion of an intra-aortic balloon pump and orotracheal intubation for mechanical ventilation were carried out in the hemodynamic laboratory and pulmonary artery catheterization was performed in the CICU following coronary angiography. These techniques were used at the operator's discretion.
Clinical eventsThe following in-hospital events were analyzed: ischemia (reinfarction and stroke), arrhythmias (atrial fibrillation, ventricular fibrillation and complete atrioventricular block), major bleeding (TIMI criteria), and mortality. Reinfarction was defined as re-elevation of troponin I above the decision limit, together with de novo symptoms or electrocardiographic findings suggestive of myocardial ischemia. Stroke was defined as focal neurological signs persisting longer than 24 hours with evidence of infarction on brain imaging (ischemic) or without (undetermined etiology).
The clinical events assessed during follow-up were all-cause mortality at 30 days and six months. No patient was lost to follow-up. Clinical data were obtained by consultation of patients’ medical records and databases or by telephone contact with the patient or relatives.
Statistical analysisCategorical variables were expressed as percentages and frequencies and compared by the chi-square test. Continuous variables were expressed as means ± standard deviation and compared using the Student's t test when appropriate (as assessed by the Shapiro-Wilks test). In-hospital events and mortality during follow-up were assessed by Kaplan-Meier analysis, statistical differences between the two groups being determined by the log rank test. Multivariate analysis using Cox proportional hazard models was performed to identify independent predictors of mortality at six months. The first model included baseline characteristics (Table 1) associated with the study's clinical event that showed p<0.10 on univariate analysis. Variables related to drug therapy in the acute phase (Table 2) and to invasive techniques and myocardial revascularization (Table 3) were added in subsequent models.
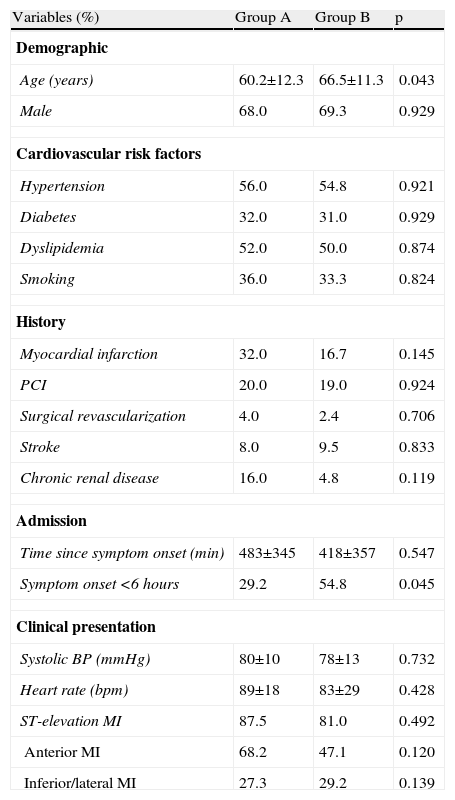
Baseline characteristics of the study population.
| Variables (%) | Group A | Group B | p |
| Demographic | |||
| Age (years) | 60.2±12.3 | 66.5±11.3 | 0.043 |
| Male | 68.0 | 69.3 | 0.929 |
| Cardiovascular risk factors | |||
| Hypertension | 56.0 | 54.8 | 0.921 |
| Diabetes | 32.0 | 31.0 | 0.929 |
| Dyslipidemia | 52.0 | 50.0 | 0.874 |
| Smoking | 36.0 | 33.3 | 0.824 |
| History | |||
| Myocardial infarction | 32.0 | 16.7 | 0.145 |
| PCI | 20.0 | 19.0 | 0.924 |
| Surgical revascularization | 4.0 | 2.4 | 0.706 |
| Stroke | 8.0 | 9.5 | 0.833 |
| Chronic renal disease | 16.0 | 4.8 | 0.119 |
| Admission | |||
| Time since symptom onset (min) | 483±345 | 418±357 | 0.547 |
| Symptom onset <6 hours | 29.2 | 54.8 | 0.045 |
| Clinical presentation | |||
| Systolic BP (mmHg) | 80±10 | 78±13 | 0.732 |
| Heart rate (bpm) | 89±18 | 83±29 | 0.428 |
| ST-elevation MI | 87.5 | 81.0 | 0.492 |
| Anterior MI | 68.2 | 47.1 | 0.120 |
| Inferior/lateral MI | 27.3 | 29.2 | 0.139 |
BP: blood pressure; MI myocardial infarction; PCI: percutaneous coronary intervention.
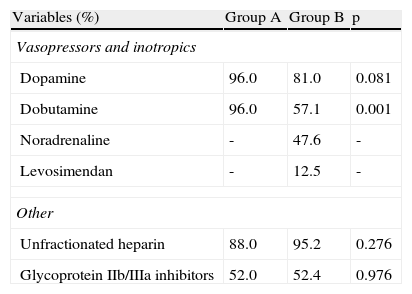
Intravenous drug therapy in the acute phase.
| Variables (%) | Group A | Group B | p |
| Vasopressors and inotropics | |||
| Dopamine | 96.0 | 81.0 | 0.081 |
| Dobutamine | 96.0 | 57.1 | 0.001 |
| Noradrenaline | - | 47.6 | - |
| Levosimendan | - | 12.5 | - |
| Other | |||
| Unfractionated heparin | 88.0 | 95.2 | 0.276 |
| Glycoprotein IIb/IIIa inhibitors | 52.0 | 52.4 | 0.976 |
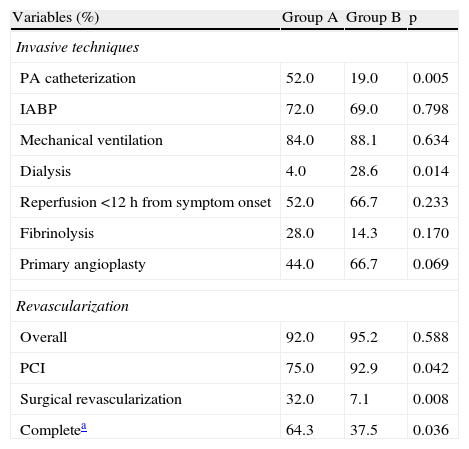
Invasive techniques and myocardial revascularization.
| Variables (%) | Group A | Group B | p |
| Invasive techniques | |||
| PA catheterization | 52.0 | 19.0 | 0.005 |
| IABP | 72.0 | 69.0 | 0.798 |
| Mechanical ventilation | 84.0 | 88.1 | 0.634 |
| Dialysis | 4.0 | 28.6 | 0.014 |
| Reperfusion <12 h from symptom onset | 52.0 | 66.7 | 0.233 |
| Fibrinolysis | 28.0 | 14.3 | 0.170 |
| Primary angioplasty | 44.0 | 66.7 | 0.069 |
| Revascularization | |||
| Overall | 92.0 | 95.2 | 0.588 |
| PCI | 75.0 | 92.9 | 0.042 |
| Surgical revascularization | 32.0 | 7.1 | 0.008 |
| Completea | 64.3 | 37.5 | 0.036 |
IABP: intra-aortic balloon pump; PA: pulmonary artery; PCI: percutaneous coronary intervention.
Results with p<0.05 were considered statistically significant. The data were analyzed using Statistical Package for the Social Sciences® for Windows, version 19.0 (SPSS, Inc., Chicago, Illinois).
ResultsCharacteristics of the study populationThe incidence of CS was 3.7% (25/683) and 4.8% (42/875) in the first and second periods, respectively (p=0.330). Group B were significantly older (66.5±11.3 vs. 60.2±12.3 years in group A, p=0.043). The proportion of patients admitted within six hours of symptom onset was also significantly higher in group B (54.8% vs. 29.2% in group A, p=0.045). Other demographic and baseline clinical characteristics did not differ significantly between the two groups (Table 1).
With regard to clinical presentation, most patients in both groups were admitted for ST-elevation MI, mainly of the anterior wall.
Diagnosis and treatmentVasopressor and inotropic agents were administered to most patients in both groups (Table 2). Use of dopamine did not differ significantly between the groups, but dobutamine was used more frequently in group A (96.0% vs. 57.1% in group B, p=0.001). Noradrenaline and levosimendan were used in group B only. Around half the patients in both groups received glycoprotein IIb/IIIa inhibitors.
Transthoracic echocardiography in the acute phase showed ejection fraction of less than 40% in most patients, with no significant difference between the groups (83.3% of group A vs. 78.6% of group B, p=0.793). Invasive techniques, reperfusion methods in the first 12 hours and myocardial revascularization procedures are shown in Table 3. There was a statistically significant reduction between the first and second periods in the proportion of patients undergoing pulmonary artery catheterization (52.0% vs. 19.0%, p=0.005) and an increase in dialysis (4.0% vs. 28.6%, p=0.014). There were no significant differences in the proportion of patients reperfused within 12 hours of symptom onset or revascularized; however, PCI was used significantly more often in group B (92.9% vs. 75.0% in group A, p=0.042), the opposite being observed for surgical revascularization (32.0% in group A vs. 7.1% in group B, p=0.008).
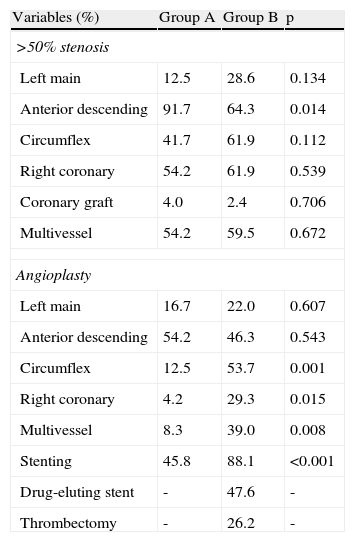
All patients underwent coronary angiography, which showed that the majority in both groups had multivessel disease (Table 4). Stenosis of the left anterior descending artery was more common in group A (91.7% vs. 64.3% in group B, p=0.014). PCI of the right coronary (29.3% vs. 4.2%, p=0.015), the circumflex artery (53.7% vs. 12.5%, p=0.001) and multiple vessels (39.0% vs. 8.3%, p=0.008), and stenting (45.8% vs. 88.1%) were more frequent in group B. The overall rate of complete revascularization was 54.5%, but was significantly higher in group A (70.8 vs. 45.2, p=0.045). Drug-eluting stents and percutaneous intracoronary thrombus aspiration were employed in group B patients only.
Angiographic features and coronary angioplasty.
| Variables (%) | Group A | Group B | p |
| >50% stenosis | |||
| Left main | 12.5 | 28.6 | 0.134 |
| Anterior descending | 91.7 | 64.3 | 0.014 |
| Circumflex | 41.7 | 61.9 | 0.112 |
| Right coronary | 54.2 | 61.9 | 0.539 |
| Coronary graft | 4.0 | 2.4 | 0.706 |
| Multivessel | 54.2 | 59.5 | 0.672 |
| Angioplasty | |||
| Left main | 16.7 | 22.0 | 0.607 |
| Anterior descending | 54.2 | 46.3 | 0.543 |
| Circumflex | 12.5 | 53.7 | 0.001 |
| Right coronary | 4.2 | 29.3 | 0.015 |
| Multivessel | 8.3 | 39.0 | 0.008 |
| Stenting | 45.8 | 88.1 | <0.001 |
| Drug-eluting stent | - | 47.6 | - |
| Thrombectomy | - | 26.2 | - |
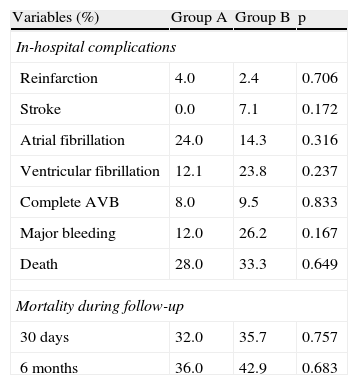
Table 5 shows in-hospital complications. There were no significant differences between the two groups in the incidence of ischemic or arrhythmic complications, major bleeding or mortality during initial hospital stay.
Clinical events.
| Variables (%) | Group A | Group B | p |
| In-hospital complications | |||
| Reinfarction | 4.0 | 2.4 | 0.706 |
| Stroke | 0.0 | 7.1 | 0.172 |
| Atrial fibrillation | 24.0 | 14.3 | 0.316 |
| Ventricular fibrillation | 12.1 | 23.8 | 0.237 |
| Complete AVB | 8.0 | 9.5 | 0.833 |
| Major bleeding | 12.0 | 26.2 | 0.167 |
| Death | 28.0 | 33.3 | 0.649 |
| Mortality during follow-up | |||
| 30 days | 32.0 | 35.7 | 0.757 |
| 6 months | 36.0 | 42.9 | 0.683 |
AVB: atrioventricular block.
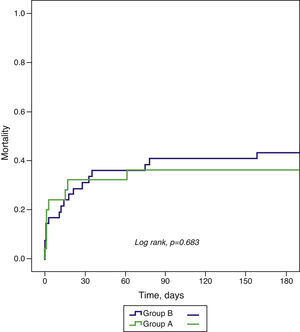
All-cause mortality at 30-day and six-month follow-up did not differ significantly between the groups (Table 5 and Figure 1).
Independent predictors of six-month mortalityIn multivariate analysis, diabetes was the only baseline characteristic that was an independent predictor of death at six months (adjusted hazard ratio [HR] 3.02; 95% confidence interval [CI] 1.38-6.60, p=0.006). Revascularized patients had lower six-month mortality (adjusted HR 0.11; 95% CI 0.03-0.42; p=0.001).
DiscussionIn this study of patients admitted for CS due to left ventricular pump failure following MI, short- and medium-term mortality did not differ significantly over a 10-year time span, despite earlier arrival at hospital and increased use of support measures and PCI in the more recent patient group. This may be because myocardial revascularization, the only intervention shown to reduce mortality in CS following MI,5 was used in a similar proportion of patients in both groups – 92.0% in group A and 95.2% in group B. In our study, myocardial revascularization was the only intervention that was independently associated with lower mortality at six months. Among baseline characteristics, diabetes was the only independent predictor of six-month mortality, and its prevalence was similar in both groups. The impact of diabetes on mortality in patients with CS has also been reported by other authors.9,10
Despite the large percentage of patients revascularized, mortality in both groups A and B was high – respectively 28.0% and 33.3% during initial hospitalization, 32.0% and 35.7% at 30 days, and 36.0% and 42.9% at six months – but similar to other contemporary series. Among patients enrolled in the US National Registry of Myocardial Infarction during the period 1995-2004, in-hospital mortality from CS also remained high, despite a reduction over time (60.3% in 1995 vs. 47.9% in 2004, p<0.001).2 In the Melbourne Interventional Group registry, 30-day mortality in CS patients undergoing coronary angioplasty between 2004 and 2007 was 36.1% in those aged under 75 and 43.2% in those aged 75 or over.9 Greenberg et al. reported six-month mortality of 52% between 2001 and 2005, and 59% between 2006 and 2011, in 170 CS patients undergoing PCI in an Israeli reference center.10
This persistently high mortality may be due to the pathophysiological mechanisms involved in the natural history of CS, which are not altered by current treatments. In CS there is a systemic inflammatory response with activation of complement, release of cytokines and increased production of NO through overexpression of inducible NO synthase.11 At the same time, despite the unquestionable benefit of myocardial revascularization in such patients, paradoxically coronary reperfusion can contribute to perpetuating CS due to reperfusion injury. Various mechanisms may be involved in such injury, such as distal microembolization, leukocyte infiltration, generation of oxygen free radicals and activation of the complement system, thus worsening microvascular damage.12
In our study, the more recent patient group was treated by PCI and stenting more often than the group admitted between 1998 and 2001. Drug-eluting stents and intracoronary thrombus aspiration were employed only in those treated between 2008 and 2011 since these techniques were not available during the earlier period. Increased use of PCI resulted in a reduction in surgical revascularization, even though the center has a cardiac surgery department. These findings are similar to those reported in other studies of MI patients with CS.2,9,10 Multivessel angioplasty was used significantly more frequently in patients treated between 2008 and 2011, in accordance with current guidelines,7 with a significant increase in PCI of the right coronary and circumflex arteries. Nevertheless, the rate of complete revascularization was higher in the earlier period due to the higher rate of surgical revascularization in these patients. It should be noted that there have been no randomized studies demonstrating the prognostic benefit of treating all angiographically significant lesions by PCI. For example, in the SHOCK trial, survival of patients undergoing complete surgical revascularization was similar irrespective of the number of vessels treated, while in those in whom only the culprit lesion were treated by PCI, mortality increased in proportion to the number of vessels with angiographically significant lesions,13 which suggests that complete revascularization confers potential benefits. However, in the recently published EHS-PCI registry, multivessel revascularization was not associated with lower mortality.14
Stead and Ebert first demonstrated in 1942 that the systemic manifestations of CS are due to left ventricular pump failure, characterized clinically by marked reduction in cardiac output and tissue hypoxia.15 Over 70 years after this initial description, much remains to be clarified concerning the management of CS, and its prognosis has remained largely unchanged throughout this period.2 Given the complex pathophysiological mechanisms involved in CS, many of the therapies developed and tested in these critically ill patients have shown no beneficial impact on prognosis.
Pulmonary artery catheterization by Swan-Ganz catheter, traditionally used for hemodynamic assessment to guide therapy, is not associated with lower mortality.16,17 Controversy surrounding the benefit of this procedure has resulted in a decrease in its use over time, from 52% to 19% in our study population, which is in agreement with other series.18
Intra-aortic balloon pump counterpulsation was used in around 70% of patients from both periods. Although use of circulatory support in the context of left ventricular pump failure seems intuitive, its benefit has not been demonstrated in randomized clinical trials.19 Left ventricular assist devices have been shown to provide greater improvement in hemodynamic parameters than intra-aortic balloon pump counterpulsation, but have no impact on mortality.20
In our study, the need for renal support therapy increased between the two periods (4.0% of patients in group A vs. 28.6% in group B). Apart from its role in treating water and salt retention, continuous veno-venous hemofiltration helps to correct metabolic acidosis, which has harmful negative inotropic and vasodilatory effects, and also removes inflammatory cytokines.21
Although NO has been shown to be involved in CS, the promising results obtained in preliminary studies on inhibiting its release were not validated in subsequent randomized trials, such as the TRIUMPH trial, in which tilarginine, a non-selective NO synthase inhibitor, did not reduce mortality in CS patients.22 Another intervention that could have prognostic impact in these patients is therapeutic hypothermia, which limits the systemic inflammatory response following cardiopulmonary arrest, by reducing the release of oxygen free radicals and pro-inflammatory cytokines in CS, among other effects.23 Prospective randomized studies will be required to confirm this hypothesis. With regard to inotropic and vasopressor support, which is essential for clinical and hemodynamic stabilization of patients with left ventricular pump failure, drugs less frequently used in this context, such as levosimendan, may be safe and efficacious alternatives.24 Noradrenaline and levosimendan were used in the more recent patient group, but in the inclusion period of the earlier group indications for use of the former were still the subject of debate and the latter was not available.25–27 In addition, there was a reduction in use of dobutamine, in favor of amines with vasoconstrictive action.
Among the present study's limitations is the fact that it was performed in a tertiary center, which may have led to selection bias. More critical patients may have died before being transferred, especially in the earlier patient group, in whom the delay between symptom onset and admission was longer. In the SHOCK trial, patients transferred to tertiary hospitals presented lower mortality than those admitted directly to the specialist centers included in the trial.5 Other limitations include the retrospective nature of the analysis, the small number of patients included, the fact that it was a single-center study, and the difficulty in establishing with any precision the time of onset of shock and the hemodynamic parameters at diagnosis.
ConclusionsCardiogenic shock due to left ventricular pump failure following MI is associated with high mortality, despite widespread use of myocardial revascularization. In the present study of patients with MI complicated by CS, mortality remained unchanged over a 10-year time span, despite an increased proportion of patients admitted within six hours of symptom onset, and greater use of PCI and support therapies. This finding probably reflects the fact that the pathophysiology of CS remains unclear, and current therapeutic options are unable to control the mechanisms involved in its genesis and perpetuation. It is thus essential to continue the search for new treatments that will improve the survival of patients with MI complicated by CS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Dores H, Ferreira J, Costa F, et al. Choque cardiogénico no enfarte agudo do miocárdio: o que mudou nos últimos 10 anos? Rev Port Cardiol. 2013;32:673–680.