Transesophageal echocardiography (TEE) is crucial in order to assess aortic anatomy after stroke. Although routinely used to assess cardiovascular anatomy and function, three-dimensional echocardiography (3D TEE) is less used for aortic evaluation. We thus sought to assess prospectively whether additional information on aortic plaque morphology could be obtained with 3D TEE after an ischemic stroke.
MethodsPatients within one week of a stroke (confirmed by brain computed tomography/magnetic resonance) underwent TEE and 3D findings were compared with two-dimensional (2D) (aorta plaque number, dimensions, area and the presence of debris and ulcerations). Patients were followed for two years for death or a new stroke.
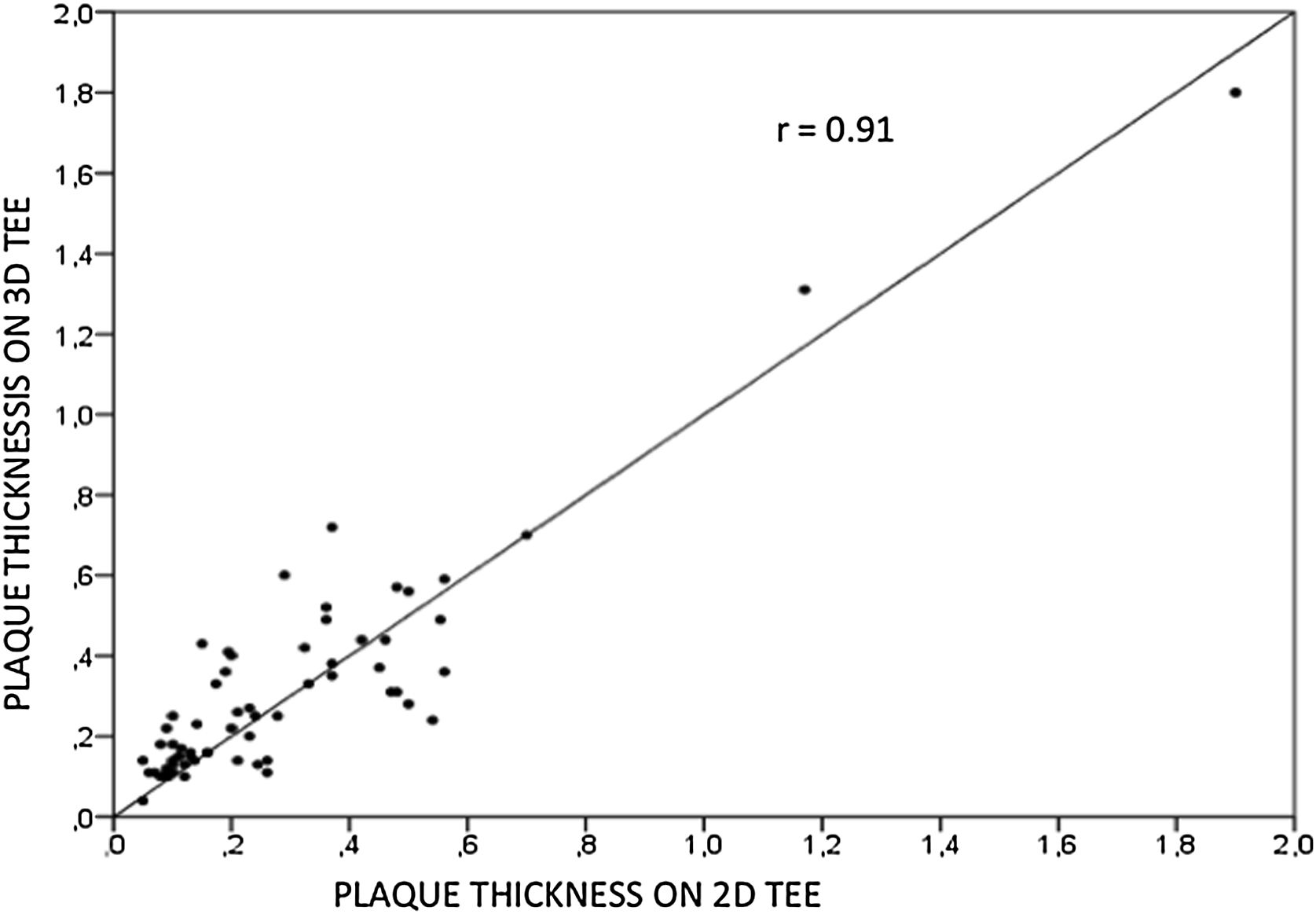
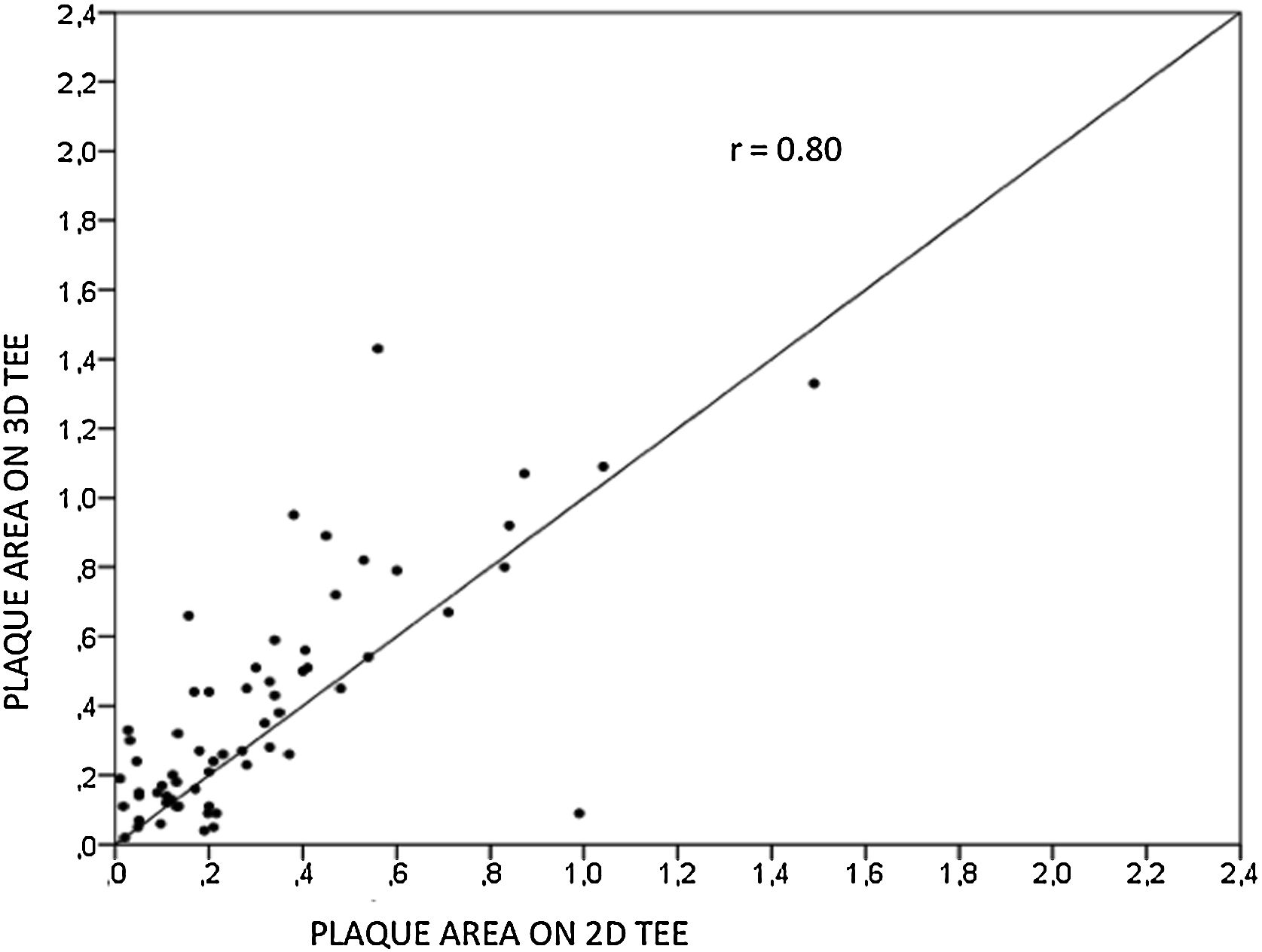
ResultsWe assessed 78 patients, 43 (55%) male, aged 62±14 years old, 92% in sinus rhythm. Aortic atheroma was found mainly in the descending aorta (50%); plaque thickness was similar for 2D TEE (0.29±0.03 cm) and 3D TEE (0.29±0.04 cm), whereas plaque area was slightly increased for 3D measurements (0.24±0.02 cm2 versus 0.37±0.03 cm2 respectively, p<0.05), with a strong correlation found both for aortic plaque thickness (r=0.91) and area (r=0.80) measurements. While aortic debris were equally seen with both techniques, 3D TEE defines the presence of ulcerations (six ulcerations unseen with 2D TEE better, p=0.03). There were 11 events (six deaths and five new strokes) during follow-up, unrelated to plaque characteristics.
ConclusionTo evaluate aortic plaque morphology, 3D TEE is superior to 2D TEE due to improved detection of ulcerated aortic plaque; this might provide additional information in patients after ischemic stroke.
O ecocardiograma transesofágico (ETE) é fundamental para avaliação da anatomia aórtica após acidente vascular cerebral (AVC) isquémico; embora frequentemente utilizado para avaliação da anatomia cardíaca, o ETE tridimensional (ETE3D) é menos usado para estudo da aorta; assim, avaliamos prospetivamente se o ETE3D adicionaria informações ao ETE bidimensional (2D) sobre a morfologia do ateroma aórtico.
MétodosPacientes com uma semana de AVC (confirmados por tomografia de crânio/ressonância magnética) foram submetidos a ETE para comparação de achados morfológicos da aorta (número de placas, dimensões, presença de debris e ulceração) pelo ETE2D e 3D. Os pacientes foram seguidos por dois anos para eventos (morte e/ou novo AVC).
ResultadosEstudamos 78 pacientes, 43 (55%) homens, idade de 62±14 anos. Ateroma aórtico foi encontrado mais frequentemente na aorta descendente (50%); a espessura da placa foi semelhante entre ETE2D e 3DTEE (0,29±0,03 cm versus 0,29±0,04 cm, p=NS), com área maior para medidas 3D (0,24±0,02 cm2versus 0,37±0,03 cm2 respetivamente, p<0,05) e excelente correlação para espessura (r=0,91) e área (r=0,80). Debris foram vistos igualmente por ambas as técnicas, porém ulcerações foram mais frequentes pelo ETE3D (seis ulcerações não visualizadas pelo ETE2D, p=0,03). No seguimento, houve 11 eventos (seis óbitos e cinco AVC), sem relação com características da placa.
ConclusãoO ETE 3D é superior ao 2D para avaliação da morfologia da placa aórtica em pacientes com AVC, em função da melhor visualização de ulcerações, podendo adicionar informações a este grupo.
The presence of atherosclerotic plaque in the thoracic aorta is a marker of ischemic cardiovascular events, including death and ischemic stroke.1 Transesophageal echocardiography (TEE) is the modality of choice to assess aortic plaque2; complex atheromas (plaques with >4 mm thickness or with plaque rupture and mobile fragments, and ulcerated plaques) are more likely to be associated with embolic events.3 Three-dimensional echocardiography (3D TEE) has been used to assess cardiovascular anatomy and function4 and is more accurate regarding measurements of cardiac volumes; 3D TEE has been extensively used to evaluate cardiac anatomy. However, data on aortic anatomy with 3DTEE are scarce,5–7 with some reports related to aortic dissection.8,9
ObjectiveWe sought to evaluate whether additional information of atherosclerotic aortic plaque morphology could be obtained by 3DTEE and the relationship between aortic plaque and outcome in patients after an ischemic stroke.
Material and methodsWe studied patients aged >18 years old, of both sexes, after an acute (<1 week) ischemic stroke confirmed by brain computed tomography or magnetic resonance imaging (MRI), and referred for TEE for evaluation of cardiac source of emboli in a tertiary hospital between June 2013 and December 2015.
Transthoracic and transesophageal echocardiographyTransthoracic and TEE were performed in patients within one week of the index event with a commercially available machine (IE 33, Philips, Andover, MA, USA). Transthoracic echocardiography was used to evaluate cardiac morphology and function (left ventricular volumes, mass and ejection fraction, valve anatomy and function); after topical anesthesia of oropharynx with xylocaine and light sedation with intravenous midazolam (0.01 mg/kg) and fentanyl (0.01 mcg/kg), the 3D TEE probe was introduced into the esophagus with the patient in the left lateral decubitus. TEE was used to assess valve morphology and function and to observe the presence of thrombi. Agitated saline solution was used both during transthoracic and transesophageal echocardiography with and without Valsalva maneuver to assess the presence of a patent foramen ovale (PFO).
Evaluation of thoracic aorta by transesophageal echocardiographyTwo-dimensional live images of the ascending aorta, aortic arch, and descending aorta were obtained for later measurements, with focus on segments with atherosclerotic plaques; the 3D images were obtained with real time 3D zoom mode, displaying a magnified pyramidal volume, with images acquired when the intima was thickened >1 mm from 2D images. The region of interest was set at the greatest contour of the plaque, and after plaque thickness was measured, tracing plaque area was undertaken from 2D and 3D images. The gain was adjusted to sharpen the plaque's edges. Measurements were undertaken offline with a dedicated software (Qlab, Phillips, Andover, MA, USA). The number of plaques and their location in the thoracic aorta were noted, and plaque thickness (defined as the distance from the medial-adventitial border and the lumen of the aorta measured at the maximal site) and area (measured by tracing the echo dense perimeter of the plaque) were measured both using 2D and 3D echocardiography. Specific morphologic features of the plaque were identified, such as the presence of plaque debris (mobile plaque components) and ulcerations (defined as a crater on the plaque ≥2.0 mm in depth and width). Complex aortic plaques were defined as plaque measuring >4 mm, a plaque with mobile debris and/or ulcers.3
Follow-upPatients were followed for two years by phone calls or hospital records to assess for events (recurrent stroke or death).
StatisticsStatistical analysis was performed using SPSS version 24.0. Data were expressed as mean±SD or proportions; association between events and categorical variables were tested with Fisher test or Chi-square; Pearson's correlation was used to test between 3D and 2D measurements of plaque area and thickness; agreement between the two measurements (2D and 3D) was evaluated with Bland-Altman analysis. Agreement between qualitative measures was undertaken with kappa statistics. Inter and intraobserver variability for 3D and 2D measurements of plaque area was tested with intraclass correlation coefficient.
ResultsWe studied 78 consecutive patients with a recent (<1 week) stroke, most of them (51%) male, with a mean age of 62±14 years old. The age range of the studied population is shown in Table 1. Most patients (70 patients, 92%) were in sinus rhythm during the examination. All patients had the diagnosis of an ischemic stroke confirmed by brain MRI (61 patients, 62%) and/or computed tomography (48 patients, 57%); 63% of the patients had anterior circulation strokes, 22% posterior circulation, and for the remaining patients, the strokes looked embolic. Regarding risk factors, more than half of the patients had arterial hypertension; other comorbidities included diabetes, hypercholesterolemia, and coronary artery disease. Clinical characteristics and demographics of the patients are displayed in Table 2.
Demographics and comorbidities of the population after Cerebrovascular accident.
| Variable | n (%) |
|---|---|
| Sex (male) | 43 (55) |
| CHD | 11 (14) |
| Smoking | 11 (14) |
| Diabetes | 26 (33) |
| Atrial fibrillation | 6 (8) |
| Dyslipidemia | 15 (19) |
| PFO | 35 (45) |
| Hypertension | 41 (53) |
CHD: coronary heart disease; PFO: patent foramen ovale.
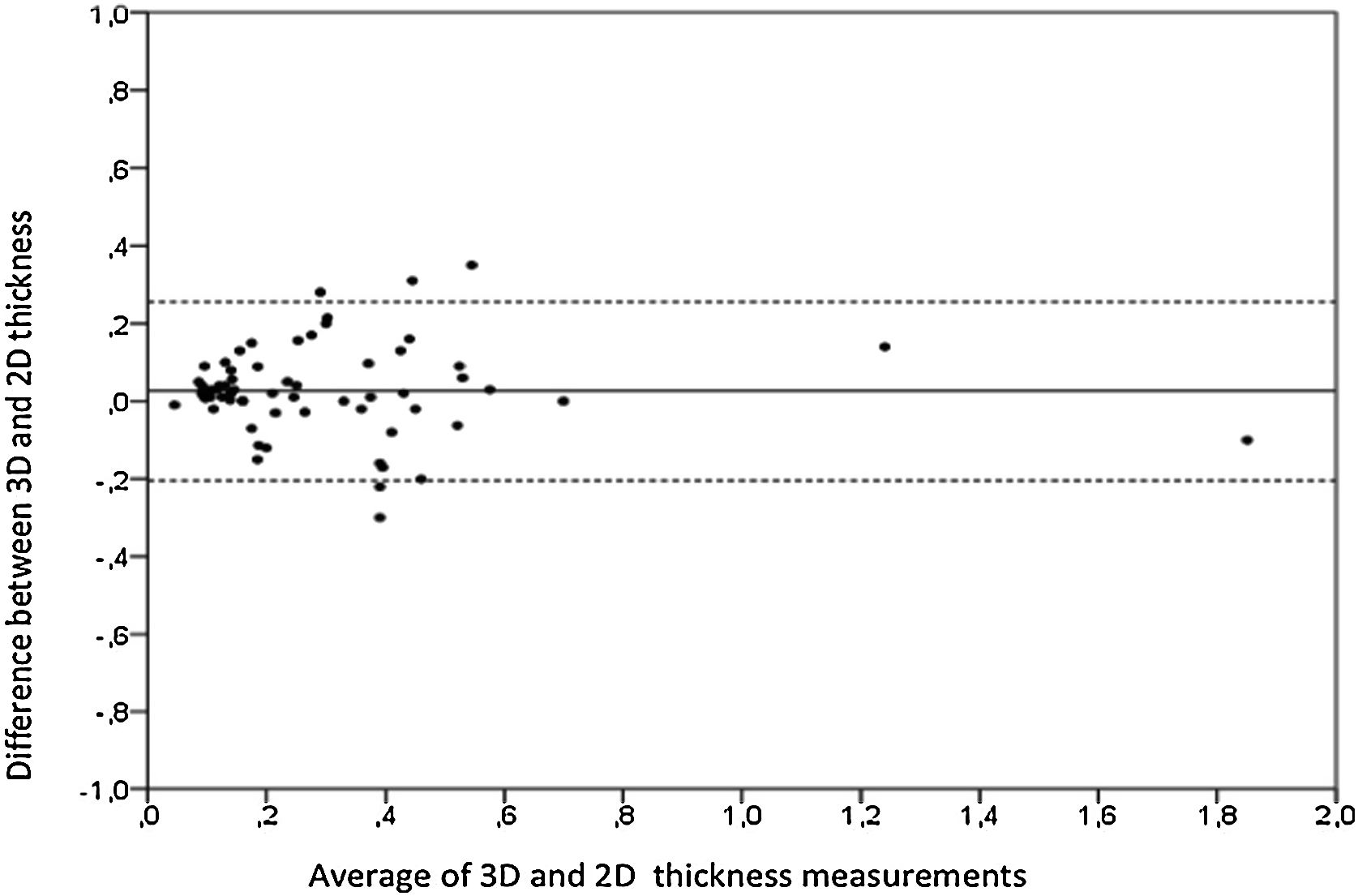
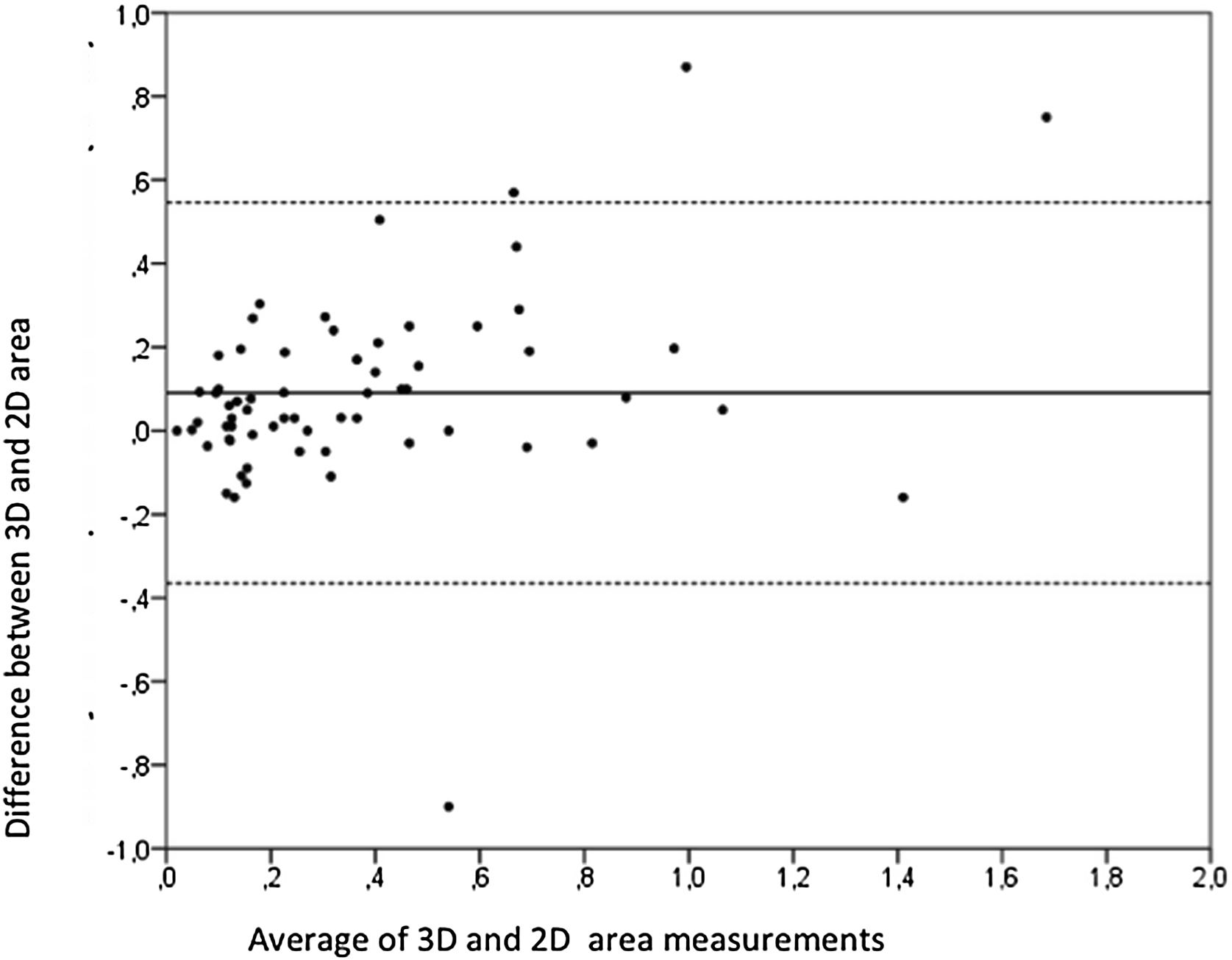
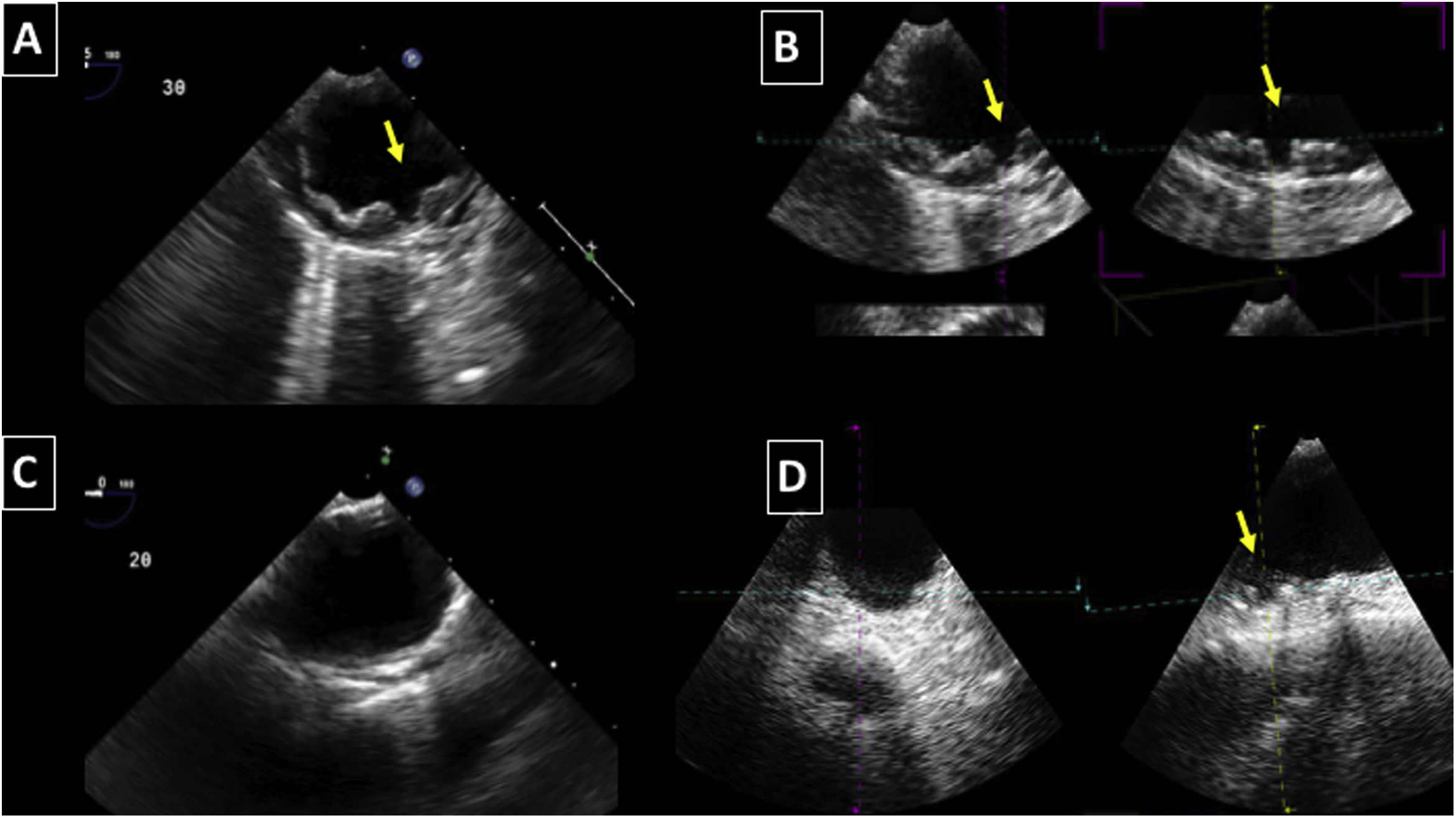
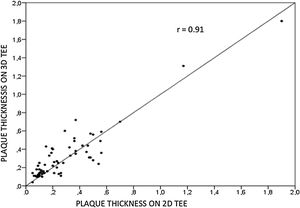
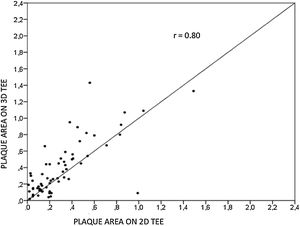
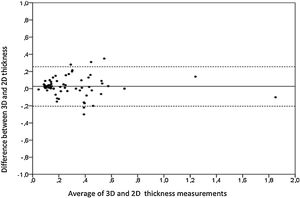
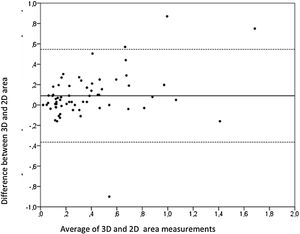
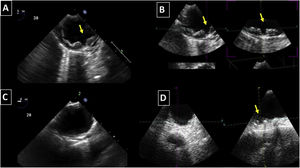
Aortic atheroma was found mainly in the descending aorta (50%) but rarely observed in the ascending aorta (2%) or aortic arch (2%). Multiple plaques were observed in the descending aorta. Measurements of aortic plaque thickness were similar for both 2D and 3D echocardiography (0.29±0.03 cm with 2D TEE and 0.29±0.04 cm with 3D TEE), whereas, for plaque area, 2D TEE measurements were slightly underestimated when compared to 3D TEE (0.24±0.02 cm2 versus 0.37±0.03 cm2 for 2D and 3D TEE respectively, p<0.05), displayed in Table 3. Correlation for aortic plaque thickness measurements and the area was very good (r=0.91 and r=0.80 respectively, Figures 1 and 2). Agreement regarding the measurements is depicted in Bland-Altman plots for both thickness and plaque area (Figures 3 and 4). Ten patients had aortic plaque ulcerations; in five of these patients, only 3D TEE identified the ulcers; in the remaining five, ulcers were detected by both methods (kappa=0.64). Images of aortic ulcerations with 2D and 3D TEE are shown in Figure 5. Regarding the number of ulcerations, 3D TEE identified 6 ulcerations, unseen with 2D TEE (one patient had two ulcerations). Regarding the mobile components of the plaque, plaque debris were observed in two patients with 2D TEE, whereas with 3D TEE, debris were seen in three patients. Altogether, 16 (20%) patients had a diagnosis of a complex aortic atheroma (aortic plaque lesions >4 mm, aortic ulcers, and debris). In six patients, it was defined by both methods, in seven diagnosis was only with 3D TEE, whereas in three patients only with 2D TEE, resulting in a modest agreement between the two methods (kappa=0.54). Discrepancies resulted from the non-visualization of one aortic plaque debris and six ulcers using 2D TEE (p<0.05). of note, for 2D TEE, in the three patients with lesions measuring >4 mm with 2D TEE but considered <4 mm with 3D TEE, the plaque was ulcerated and similarly regarded to be complex, because of the coexistence of lesions. Diagnosis of a complex plaque was improved with 3D TEE compared to 2D TEE (p<0.05).
Intra and interobserver variability were tested for 2D and 3D measurements in ten patients, both for the same observer (ACR) and a second observer (LG) and assessed with intraclass correlation testing. ICC coefficient was 0.91 for thickness (95% CI 0.86–0.94) and 0.76 for area measurements (95% CI 0.61–0.95).
Additional findingsA PFO was found in 35 (45%) patients; none had thrombus in the left atrial appendage; one patient had an aortic prosthesis thrombosis.
Follow-upAll patients were followed for two years for mortality or recurrent strokes. There were 11 (14%) patients who presented with new events: six (8%) deaths and 5 (6.4%) new episodes of CVA or a transient ischemic attack. Deaths were cardiovascular in two patients. Non-cardiac death resulted from sepsis in two patients, respiratory failure in one, and gastric carcinoma in another. A recurrent stroke was attributed to atrial fibrillation in one patient, a PFO in another, and significant artery atherosclerosis of the head and neck was in another. In two patients no clear reason for recurrence was established; malignancy was suspected to be the underlying cause. In this series, a worse outcome was associated with age >60 years (p=0.001), hypertension (0.03), and coronary heart disease (p=0.045) but unrelated to the presence of a complex plaque, ulcers or a PFO.
DiscussionThe presence of atherosclerotic plaque in the thoracic aorta has been shown to be a marker of ischemic cardiovascular events, including death and ischemic stroke.1,3 Transesophageal echocardiography (TEE) is the modality of choice to recognize and evaluate aortic plaque, with complex atheroma (plaques with >4 mm thickness, with plaque rupture, mobile fragments, and ulcerated plaques) more likely to be associated with embolic events; three-dimensional transesophageal echocardiography (3D TEE) appears to be more accurate regarding measurements of cardiac volumes4 as well as valvular function.9 However data on aortic anatomy with 3D echocardiography are scarce,7 with few reports on aortic dissection.8,10 We compared aortic plaque morphology with 2D and 3D TEE in patients after acute stroke. For plaque thickness or area, we did not find a significant difference between the measurements, with an excellent correlation for both methods (r=0.91 and r=0.80, respectively); however, less agreement was observed for 2D and 3D area measurements, according to Bland-Altman plot; this difference might be partially explained from subtle plane variations allowing for the inclusion of a more significant plaque proportion with 3D; since thickness was measured at a single point, as opposed to the area, less measurement variation was found. Additionally, three patients had borderline measurements, and the plaque was defined >4 mm by 2D TEE but considered <4 mm by 3D TEE, findings that could be regarded as 3D TEE false negative: we believe a potential explanation is the lower spatial resolution of 3DTEE. Conversely, because a plaque ulcer was concomitantly found, these patients were ultimately deemed to have a complex plaque. Alternatively, this finding contradicts another report5 that revealed atherosclerotic aortic plaques were underestimated by 2D TEE measurements, resulting in more patients classified as having a complex plaque when using 3D TEE. While we observed plaque measurements to be quite similar with both methods, we did find a higher number of complex plaques, defined by 3DTEE, in effect due to the increased diagnosis of ulcerated plaques. It has been previously shown that aortic atheroma complexity, rather than size, is more likely to be associated with cerebral ischemic events, with mobile or ulcerated plaques associated with a higher risk of stroke.11 Plaque surface morphology and ulcerations may reflect plaque vulnerability; ulcerated aortic plaques are a potential cause of embolism12 and have been considered a marker of stroke. TEE can improve visualization of ulcerated plaque since the structures are better displayed by 3D than conventional echocardiography, regarding different planes and dimensions, thus more accurately defining the ulcers. Regarding the assessment of aortic atheroma, an anecdotal report also found improved visualization of aortic ulcers with 3D TEE over 2DTEE.7
Conversely, as expected, atherosclerotic plaques were primarily seen in the descending aorta, both using 3D and 2D TEE, with few plaques observed in the aortic arch or ascending aorta; when found in the descending aorta, however, plaques are markers of stroke risk, and have been associated to all-cause mortality.13 Additionally, descending thoracic aorta atheroma has been demonstrated to be related to retrograde aortic embolization.14 As to the presence of complex aortic atheroma, these were observed in only 20% of our patients. In up to 40% of patients without an apparent reason for stroke, a complex aortic atheroma (>4 mm thick) can be detected with TEE, and these have been considered an independent risk for stroke.3 Our population, however, was younger (average around 60 years of old), perhaps contributing to the lower prevalence of aortic plaque.
Concerning the clinical impact of the TEE findings, the methods presented incremental value on patient management: in one patient with aortic mobile debris (considered to be thrombus formation demanding anticoagulation), the stroke was considered to be associated with aortic results, and the patient was anticoagulated. The decision to intervene in case a PFO was left to the attending physician. Although 35 patients showed PFO with significant shunt on TEE, a septal occluder device was implanted in only two patients. Lastly, one patient had prosthesis thrombosis, and anticoagulation was more aggressive. For aortic ulcers, traditional treatment (aspirin alone) was given.
Finally, although not within the scope of this study, it is worth briefly discussing the importance of other non-invasive imaging methods for aortic plaque evaluation. Computed tomography and MRI have been introduced to the study of atherothrombosis, allowing both direct imaging of the lesions and measurement of atherosclerotic burden, by characterization of the plaque components. MRI can provide useful information about morphology and tissue composition of the atherosclerotic plaque (size, composition and biological activity) that can be used to assess therapeutic effects of clinical studies and subclinical disease15; this examination, however, may not be promptly available in all facilities, has a few contraindications and demands a longer scan time: specially when the patient's cooperation is limited, cardiac multidetector computed tomography (MDCT) can be an alternative in the diagnosis of embolic sources, also defining atheroma morphology to predict the risk of recurrence after a ischemic stroke; compared to TEE, they can detect smaller atheromas.16 Limitations for these methods are the high cost, need for contrast use, and for CT, another drawback is the risk of high radiation exposure.17
Concerning follow-up, we confirmed the relation between conventional risk factors (hypertension, age, coronary heart disease) and events (death and CVA); this association of risk factors have been extensively studied and proved after stroke; however, probably due to the limited number of patients included, the current study was unable to support unequivocal improvement in prognostic value for 3D TEE in patients with stroke. Several factors may be involved in stroke recurrence: in our series recurrence was only 6%; this certainly could be higher with a longer follow-up, however, recurrence also depends on stroke etiology; for cardioembolic stroke, for example, recurrence is high in the first weeks decreasing during a 12-month follow-up; age is also a major influencing factor, as it increases heterogeneity. There was significant heterogeneity in our series, most likely reflecting the inclusion of a number of distinct subgroups, with low patient numbers. Moreover, the specific treatment of the stroke impacts the prognosis, contributing to fewer recurrent events during follow-up. A PFO closure has been associated with a lower risk of recurrent stroke compared with antithrombotic therapy (antiplatelet therapy or anticoagulation), and this might have influenced the results, however, a minority of patients underwent this intervention. Intensive medical treatment has been shown to modify recurrence rates of stroke with better outcomes after aggressive medical treatment.18 Secondary prophylaxis stroke treatment continues to be the aggressive management of systemic atherosclerosis (e.g., antiplatelet and lipid-lowering therapy, blood pressure and glycemic control, and smoking cessation). Some confounders, such as anticoagulant and statin use, however, cannot be completely accounted for.
Study limitationsThe interposition of the trachea between the ascending aorta and the esophagus creates a blind area that limits visualization of the distal ascending aorta and proximal arch, preventing imaging atheroma in these areas. We routinely assess the images of the aortic arch by transthoracic echocardiography, however, for the study objective (comparison of 2D and 3D images with TEE), these images were not useful. Additionally, the small number of patients is a major limitation of the study; prospective studies in larger cohorts are needed to establish stronger evidence for ulcerated plaque influence over stroke patients. Finally, we only used the 3D zoom method and not a full volume imaging to assess plaque morphology; however, we could focus on the segments with atherosclerotic plaques in the 2D scanning and decrease stitch artifacts.
ConclusionThree-dimensional TEE is superior to 2D TEE in assessing aortic plaque morphology by improving detection of atheromatous aortic ulcers; future studies should focus on assessing aortic ulcerations as they might provide additional prognostic information in patients after ischemic stroke.
Conflicts of interestThe authors have no conflicts of interest to declare.