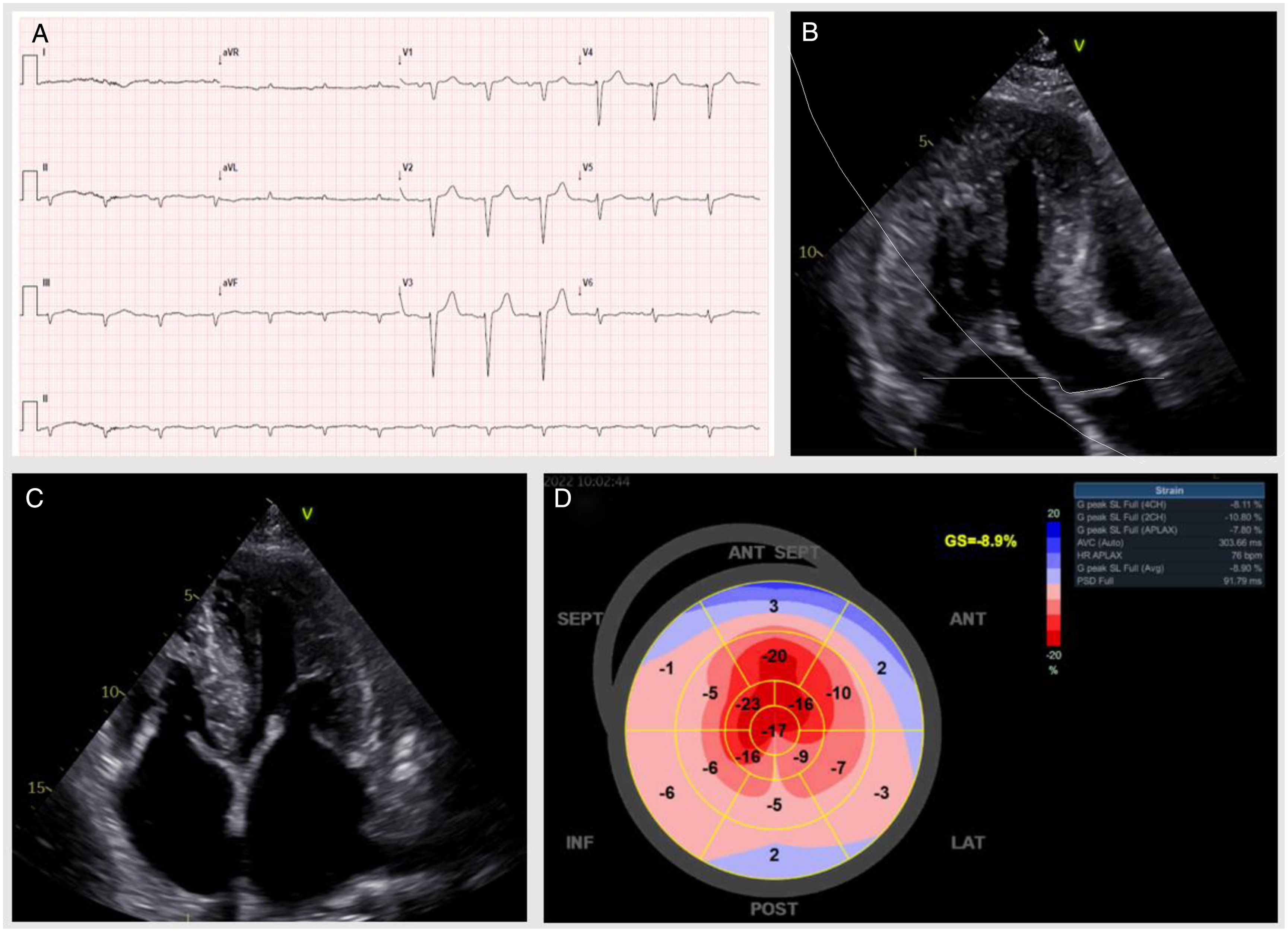
We present the case of a 66 year-old male, previously treated invasively for chronic coronary syndrome, dyslipidemia and a former smoker, who presented with de novo symptomatic heart failure (HF). The patient had a three-month history of fatigue, orthopnea, and progressive bilateral leg edema (New York Heart Association II). Physical examination was unremarkable. Initial study revealed: sinus rhythm and a poor R wave progression on precordial leads in electrocardiogram (ECG) (Figure 1A), small left ventricle (LV) with severe hypertrophy, preserved ejection fraction and a global longitudinal strain of −8.9%, with an “apical sparing” pattern on transthoracic echocardiogram (Figure 1B–D). N-terminal prohormone of brain natriuretic peptide of 8643 pg/mL and plasma creatinine of 1.3 mg/dL. Cardiac magnetic resonance (CMR) further confirmed these findings, revealing a diffuse late gadolinium enhancement with a subendocardial distribution throughout the LV. Altogether, these findings were highly suggestive of cardiac amyloidosis (CA); therefore, the patient underwent further workup. The [99mTc]Tc-HMDP bone scintigraphy revealed no myocardial uptake – a Perugini score of 0 (Figure 2A). Transthyretin (TTR) genetic testing was negative. AL amyloidosis was then suspected, especially given the presence of a monoclonal peak and serum and urine immunofixation positivity for IgG lambda. However, additional assessment, including myelogram and bone marrow biopsy, supported low-risk monoclonal gammopathy of undetermined significance (MGUS) as the likely culprit of the aforementioned findings.
Electrocardiogram and transthoracic echocardiogram. (A) Sinus rhythm, with a heart rate of 78 bpm and poor progression of R waves in precordial leads. (B–D) Small LV cavity (end-diastolic index volume 40 mL/m2) with severe hypertrophy (LV mass index 222 g/m2, septal maximum thickness 22 mm); LV ejection fraction 55–60% and global longitudinal strain −8.9%, exhibiting “apical sparing” (apical/medium-basal ratio of 3.5). Diastolic evaluation: restrictive pattern; E/e′ 20 and tissue velocities <6 cm/s. Left atrium slightly dilated. Right chambers non-dilated and right ventricle (RV) with a reduced systolic function (TAPSE 12 mm, RV S′ 0.12 m/s). Estimated systolic pulmonary arterial pressure was 56 mmHg.
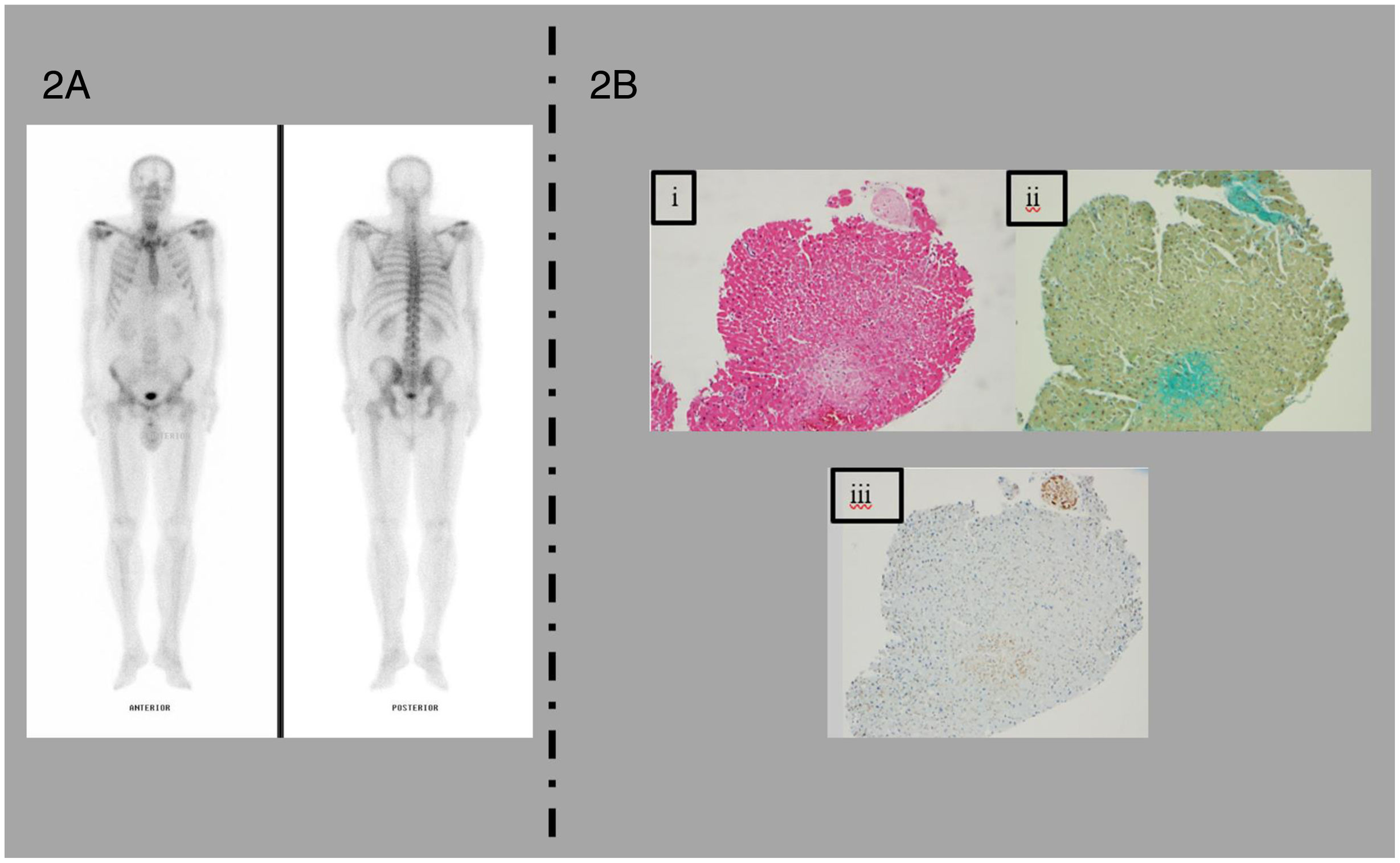
[99mTc]Tc-HMDP bone scintigraphy and histopathological findings in endomyocardial biopsy. (A) Results of the planar whole-body scintigraphy with [99mTc]Tc-HMDP showing no cardiac uptake (Perugini score=0). (B) i – Cardiac amyloidosis – pale pink, extracellular, glassy, hyaline material on H&E staining. ii – Sodium sulphate-Alcian blue method showing green staining in the extracellular matrix of the myocardium. iii – Immunohistochemical study showing positivity for TTR. No light or heavy chains of immunoglobulins were observed.
In the multidisciplinary team discussion, we decided to perform an endomyocardial biopsy (EMB), given the clinical picture of de novo heart failure with preserved ejection fraction (HFpEF) with a hypertrophic phenotype, imaging findings highly suggestive of CA and higher diagnostic yield. The histopathology was positive for amyloid, and only TTR was detected on immunohistochemistry (Figure 2B). Mass spectrometry was inconclusive (TTR was present in small amounts and other amyloid proteins were present) and failed to identify light or heavy chains of immunoglobulins. Given the above studies and after discussion with hematology, a presumptive diagnosis of biopsy-proven wild-type TTR amyloid cardiomyopathy (ATTR-CM) was made, with concurrent IgG MGUS. The patient was deemed a candidate for and started on disease-modifying treatment (tafamidis 61 mg); however, treatment response was rather poor, with rapidly worsening HF, culminating in death 6 months after the initial diagnosis.
This case highlights the importance of maintaining a high clinical suspicion and using EMB in suspected CA cases with inconclusive non-invasive tests. It also emphasizes the need to exclude AL amyloidosis and consider histological diagnosis when non-invasive criteria are not met, particularly with low or absent myocardial uptake in bone scintigraphy.1
Blood and urine monoclonal protein studies were not reliable predictors of cardiac amyloid type in patients undergoing EMB, with a sensitivity of 74% and specificity of 75%.2 In this case, invasive testing confirmed the diagnosis of IgG MGUS. EMB immunohistochemistry showed positive results for TTR, but mass spectrometry, a high sensitivity and specificity method, was inconclusive, failing to identify free chain immunoglobulins. Therefore, a definitive diagnosis could not be established with certainty.
To the best of our knowledge, this is the second reported case of wild-type ATTR-CM with negative bone scintigraphy. Similar to a previously reported case, our patient presented with non-specific signs of HF. The diagnosis of ATTR-CM is often delayed due to confounding comorbidities such as coronary artery disease. Delayed diagnosis can limit the effectiveness of disease-modifying treatment.3
False negatives in bone scintigraphy can delay or miss the diagnosis of ATTR-CM.3,5 Different radiotracers have varying accuracy in detecting ATTR-CM. Also, mutated forms of TTR may have reduced avidity for radiotracers (e.g. Ser77Tyr, Phe64Leu and Val50Met mutations),5 leading to higher rates of false negatives. Indeed, the [99mTc]Tc-DPD bone scintigraphy may have higher rates of “false negatives” in the Val50Met (Portuguese) mutation, and, possibly Phe64Leu mutation.4,5 The lack of sensitivity may be due to the composition and conformation of amyloid fibrils, affecting radiotracer binding.5 Type A amyloid fibrils show significant myocardial uptake, while type B amyloid fibrils do not. The exact explanation for the lack of sensitivity remains unclear.5
Another possible explanation for false negatives in bone scintigraphy is the presence of initial amyloidosis with deposits below the detection threshold, reducing imaging accuracy.4 Previous reports question the generalization of a 100% positive predictive value.
Musumeci et al.4 emphasized a possible reduced sensitivity of bone scintigraphy in patients with CA and Phe64Leu TTR, of whom 65% had low or absent myocardial uptake. However, only two patients had a biopsy-proven ATTR-CM diagnosis. Multimodal approach comprising CMR and EMB should be considered in such patients.4 The case herein reported additionally supports this argument, further extending it to wild-type ATTR-CM.
We have described the case of a patient with biopsy-proven wild-type ATTR-CM presenting with a negative bone scintigraphy and diagnosis of monoclonal gammopathy further complicating the diagnostic workup. Similar scenarios have rarely been reported. Our findings emphasize the importance of invasive CA workup in a patient in whom non-invasive testing would suggest the opposite, even in the presence of a monoclonal gammopathy.
Conflicts of interestThe authors have no conflicts of interest to declare.


![[99mTc]Tc-HMDP bone scintigraphy and histopathological findings in endomyocardial biopsy. (A) Results of the planar whole-body scintigraphy with [99mTc]Tc-HMDP showing no cardiac uptake (Perugini score=0). (B) i – Cardiac amyloidosis – pale pink, extracellular, glassy, hyaline material on H&E staining. ii – Sodium sulphate-Alcian blue method showing green staining in the extracellular matrix of the myocardium. iii – Immunohistochemical study showing positivity for TTR. No light or heavy chains of immunoglobulins were observed. [99mTc]Tc-HMDP bone scintigraphy and histopathological findings in endomyocardial biopsy. (A) Results of the planar whole-body scintigraphy with [99mTc]Tc-HMDP showing no cardiac uptake (Perugini score=0). (B) i – Cardiac amyloidosis – pale pink, extracellular, glassy, hyaline material on H&E staining. ii – Sodium sulphate-Alcian blue method showing green staining in the extracellular matrix of the myocardium. iii – Immunohistochemical study showing positivity for TTR. No light or heavy chains of immunoglobulins were observed.](https://static.elsevier.es/multimedia/08702551/0000004300000003/v2_202403080630/S087025512300464X/v2_202403080630/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)

