One-year mortality after hospitalization for heart failure (HF) is high. This study aims to identify predictive factors of one-year mortality.
MethodsThis is a retrospective, single-center and observational study. All patients hospitalized for acute HF during one year were enrolled.
ResultsA total of 429 patients were enrolled, mean age of 79 years. The in-hospital and one-year all-cause mortality rates were 7.9% and 34.3%, respectively. In the univariable analysis, the factors significantly associated with higher one-year mortality risk were: age ≥80 years (odds ratio (OR)=2.05, 95% confidence interval (CI) 1.35–3.11, p=0.001); active cancer (OR=2.93, 95% CI 1.36–6.32, p=0.008); dementia (OR=2.84, 95% CI 1.81–4.47, p<0.001); functional dependency (OR=2.63, 95% CI 1.65–4.19, p<0.001); atrial fibrillation (OR=1.86, 95% CI 1.24–2.80, p=0.004); higher creatinine (OR=2.03, 95% CI 1.29–3.21, p=0.002), urea (OR=2.92, 95% CI 1.95–4.36, p<0.001) and red cell distribution width (RDW; 4thQ OR=5.59, 95% CI 3.03–10.32, p=0.001); and lower hematocrit (OR=0.94, 95% CI 0.91–0.97, p<0.001), hemoglobin (OR=0.83, 95% CI 0.75–0.92, p<0.001) and platelet distribution width (PDW; OR=0.89, 95% CI 0.82–0.97, p=0.005). In the multivariable analysis, the independent predictors of higher one-year mortality risk were: age ≥80 years (OR=2.05, 95% CI 1.21–3.48); active cancer (OR=2.70, 95% CI 1.03–7.01); dementia (OR=2.69, 95% CI 1.53–4.74); higher urea (OR=2.97, 95% CI 1.84–4.80) and RDW (4thQ OR=5.24, 95% CI 2.55–10.76); and lower PDW (OR=0.88, 95% CI 0.80–0.97).
ConclusionsActive cancer, dementia, and high values for urea and RDW at admission are predictors of one-year mortality in patients hospitalized for HF. These variables are readily available at admission and can support the clinical management of HF patients.
A mortalidade no primeiro ano após a hospitalização por insuficiência cardíaca é alta. Este estudo tem como objetivo identificar fatores preditivos de mortalidade nesse período.
MétodosEste é um estudo retrospetivo e observacional de um só centro. Todos os doentes hospitalizados por insuficiência cardíaca aguda durante o período de um ano foram incluídos.
ResultadosForam incluídos 429 doentes com idade média de 79 anos. As taxas de mortalidade intra-hospitalar e por todas as causas após um ano foram de 7,9% e 34,3%, respetivamente. Na análise univariável, os fatores significativamente associados com maior risco de mortalidade a um ano foram: idade ≥ 80 anos (OR=2,05, IC 95% 1,35-3,11, p=0,001); neoplasia ativa (OR=2,93, IC 95% 1,36-6,32, p=0,008); demência (OR=2,84, IC 95% 1,81-4,47, p<0,001); dependência funcional (OR=2,63, IC 95% 1,65-4,19, p<0,001); fibrilhação auricular (OR=1,86, IC 95% 1,24-2,80, p=0,004); valores elevados de creatinina (OR=2,03, IC 95% 1,29-3,21, p=0,002), ureia (OR=2,92, IC 95% 1,95-4,36, p<0,001) e índice de distribuição de eritrócitos (RDW; 4Q OR=5,59, IC 95% 3,03-10,32, p=0,001); e valores baixos de hematócrito (OR=0,94, IC 95% 0,91-0,97, p<0,001), hemoglobina (OR=0,83, IC 95% 0,75-0,92, p<0,001) e índice de distribuição de plaquetas (PDW; OR=0,89, IC 95% 0,82-0,97, p=0,005). Na análise multivariável, os preditores independentes de maior risco de mortalidade a um ano foram: idade ≥ 80 anos (OR=2,05, IC 95% 1,21-3,48); neoplasia ativa (OR=2,70, IC 95% 1,03-7,01); demência (OR=2,69, IC 95% 1,53-4,74); ureia elevada (OR=2,97, IC 95% 1,84-4,80) e RDW (4Q OR=5,24, IC 95% 2,55-10,76); e PDW baixo (OR=0,88, IC 95% 0,80-0,97).
ConclusõesNeoplasia ativa, demência e valores elevados de ureia e RDW na admissão são preditores de mortalidade após um ano dos doentes hospitalizados por insuficiência cardíaca. Estes dados estão facilmente disponíveis na admissão e podem auxiliar na gestão clínica de doentes com insuficiência cardíaca.
Heart failure (HF) is a prevalent condition associated with high mortality and morbidity and reduced quality of life.1 Comorbidities are frequent among HF patients, leading to increased hospitalization rates and mortality. The prevalence of comorbidities is higher in patients with more severe signs of HF.2 According to international reports, one-year mortality and re-hospitalization rates for HF patients are 17% and 44%, respectively.3,4 HF is the most common reason for hospitalization in people >65 years in economically developed countries.5
Little is known about the long-term prognosis and risk factors for a fatal outcome in patients hospitalized with acute HF (AHF).6 The increasing incidence and associated morbidity and mortality of AHF mean there is an urgent need to better understand this population.7 Risk stratification of HF patients is important to identify high-risk patients who may benefit from advanced treatments. Besides improving risk stratification, identifying new prognostic markers may also provide information about the pathophysiology of HF and support the clinical management of HF patients and decision-making as to the best therapeutic strategy.
This study reports the impact on one-year mortality of comorbidities and biochemical parameters of patients hospitalized for HF during one-year at an Internal Medicine Department of a large tertiary university hospital in Porto, Portugal.
MethodsStudy design and clinical settingThis is a retrospective, single-center, observational study. The study design and detailed characterization of the study population have been described elsewhere.8 In short, all patients discharged from the Internal Medicine Department of Centro Hospitalar Universitário de Santo António, Porto, Portugal, over a one-year period were considered for enrollment. Based on the discharge summary review, patients were selected for enrollment if they had been hospitalized for AHF, either de novo or due to chronic decompensation. HF was diagnosed according to the European Society of Cardiology guidelines available at the time of study enrollment.9 All patients without an echocardiogram presented the clinical syndrome of HF, relevant cardiac disease documented in their clinical record, and N-terminal-pro-B type natriuretic peptide (NT-proBNP) values >1000 pg/mL. For the purposes of this study, the first hospitalization for AHF during the one-year study period was considered the index hospitalization.
Data collectionData was collected based on the hospital discharge summaries, clinical records, and telephone interviews. The following data were collected from the discharge summary: sex, age, risk factors for HF, comorbidities, functional status, and HF etiology. In addition, biochemical parameters and systolic blood pressure were collected from the admission clinical records and laboratory data records.
Comorbidities and conditions considered for analysis were hypertension, diabetes (DM), cerebrovascular disease, peripheral arteriopathy, atrial fibrillation (AF), active cancer, chronic lung disease, sleep disorder, and/or hypoventilation syndrome, dementia, and functional dependency. The occurrence of comorbidities was ascertained by the investigators through the critical analysis of each diagnosis after clinical record review and was considered if diagnosed before or at the index hospitalization.
Hospital admission biochemical parameters considered for analysis were blood plasma creatinine, urea, potassium, sodium, hemoglobin, hematocrit, platelet count, platelet distribution width (PDW), and red cell distribution width (RDW).
Other clinical records were reviewed for biometric, echocardiogram, follow-up, and re-hospitalization data. The etiology of cardiac disease was established through clinical data and echocardiogram. Only re-hospitalizations at the study center were considered, given the difficulty of accessing patients’ health data in other public or private health organizations, which could underestimate re-hospitalizations. Mortality data were obtained from clinical records and telephone interviews.
OutcomeThe main outcome was to characterize the factors associated with mortality in patients hospitalized for HF one-year after discharge from the index admission.
Study oversightL.T. and D.M. performed the statistical analysis, I.M. and R.L.R. prepared the manuscript, and all authors reviewed and approved the final version of the manuscript. All authors vouch for the accuracy and completeness of the data and analysis.
Statistical analysisCategorical variables are presented as percentages, while continuous variables are presented as means and standard deviation (SD) plus median and interquartile range (IQR). Differences in mortality related to clinical parameters and other factors were first analyzed using the Chi-Square test and T-test for categorical and continuous variables, respectively. Independent predictors of mortality within the first year after discharge were analyzed using multivariable logistic regression. The final multivariable model includes all the variables that remain statistically significant after adjusting for the remaining variables. This study reports the one-year follow-up outcomes for all patients. Statistical analysis was performed using SPSS for Windows, version 22.
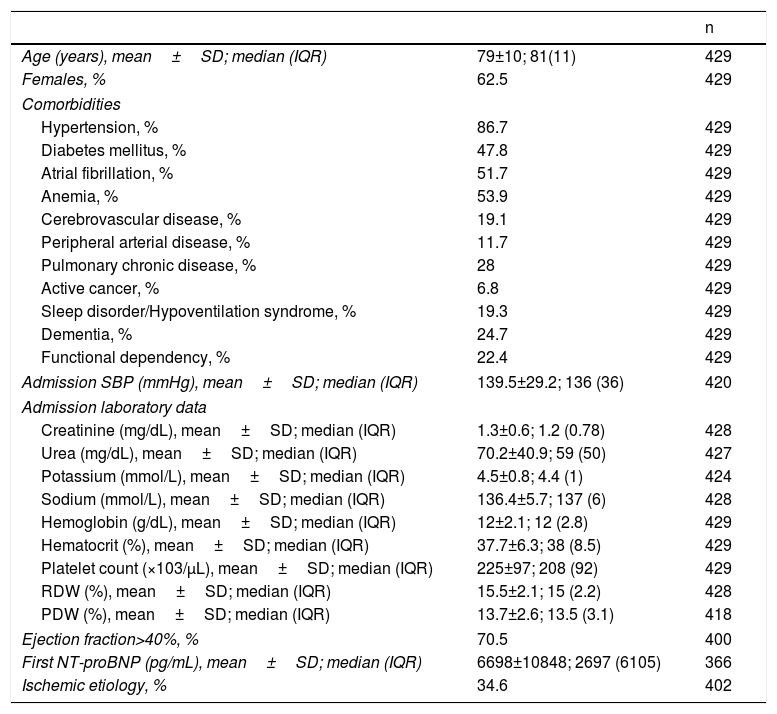
ResultsPatients and characteristicsA total of 429 patients were enrolled in this study, hospitalized for AHF, with an average stay of 11.7 days (median 10 days, range 1–117 days). The mean age was 79 years, and 62.5% were women. Ninety-six (22.4%) patients were functionally dependent for basic activities of daily living. Hypertension was the most prevalent comorbidity (86.7%), and 31.9% of all patients had more than three comorbidities.8 Echocardiogram was available in 400 patients, and 70.5% of them had left ventricular ejection fraction (LVEF)>40%. In the 29 patients who did not have a detailed echocardiogram report, kidney disease did not seem to be responsible for the high values of NT-proBNP, as only four of these patients presented plasma creatinine levels >1.5 mg/dL. Ischemic etiology for HF was present in 34.6% of patients. Table 1 summarizes the baseline characteristics of the study cohort.
Baseline characteristics of the study cohort.
| n | ||
|---|---|---|
| Age (years), mean±SD; median (IQR) | 79±10; 81(11) | 429 |
| Females, % | 62.5 | 429 |
| Comorbidities | ||
| Hypertension, % | 86.7 | 429 |
| Diabetes mellitus, % | 47.8 | 429 |
| Atrial fibrillation, % | 51.7 | 429 |
| Anemia, % | 53.9 | 429 |
| Cerebrovascular disease, % | 19.1 | 429 |
| Peripheral arterial disease, % | 11.7 | 429 |
| Pulmonary chronic disease, % | 28 | 429 |
| Active cancer, % | 6.8 | 429 |
| Sleep disorder/Hypoventilation syndrome, % | 19.3 | 429 |
| Dementia, % | 24.7 | 429 |
| Functional dependency, % | 22.4 | 429 |
| Admission SBP (mmHg), mean±SD; median (IQR) | 139.5±29.2; 136 (36) | 420 |
| Admission laboratory data | ||
| Creatinine (mg/dL), mean±SD; median (IQR) | 1.3±0.6; 1.2 (0.78) | 428 |
| Urea (mg/dL), mean±SD; median (IQR) | 70.2±40.9; 59 (50) | 427 |
| Potassium (mmol/L), mean±SD; median (IQR) | 4.5±0.8; 4.4 (1) | 424 |
| Sodium (mmol/L), mean±SD; median (IQR) | 136.4±5.7; 137 (6) | 428 |
| Hemoglobin (g/dL), mean±SD; median (IQR) | 12±2.1; 12 (2.8) | 429 |
| Hematocrit (%), mean±SD; median (IQR) | 37.7±6.3; 38 (8.5) | 429 |
| Platelet count (×103/μL), mean±SD; median (IQR) | 225±97; 208 (92) | 429 |
| RDW (%), mean±SD; median (IQR) | 15.5±2.1; 15 (2.2) | 428 |
| PDW (%), mean±SD; median (IQR) | 13.7±2.6; 13.5 (3.1) | 418 |
| Ejection fraction>40%, % | 70.5 | 400 |
| First NT-proBNP (pg/mL), mean±SD; median (IQR) | 6698±10848; 2697 (6105) | 366 |
| Ischemic etiology, % | 34.6 | 402 |
IQR: interquartile range; PDW: platelet distribution width; RDW: red cell distribution width; SBP: systolic blood pressure; SD: standard deviation.
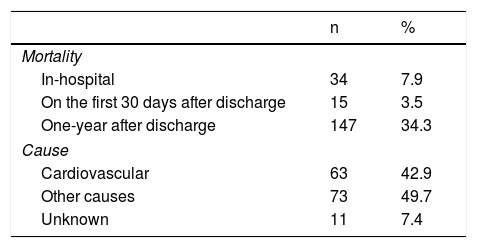
The in-hospital and one-year all-cause mortality rates were 7.9% and 34.3%, respectively. One patient was lost to follow-up after discharge. Mortality data are summarized in Table 2. During the first year of follow-up after discharge, 33.2% of the 394 patients discharged alive and with follow-up data were re-hospitalized for HF.
Mortality data.a
| n | % | |
|---|---|---|
| Mortality | ||
| In-hospital | 34 | 7.9 |
| On the first 30 days after discharge | 15 | 3.5 |
| One-year after discharge | 147 | 34.3 |
| Cause | ||
| Cardiovascular | 63 | 42.9 |
| Other causes | 73 | 49.7 |
| Unknown | 11 | 7.4 |
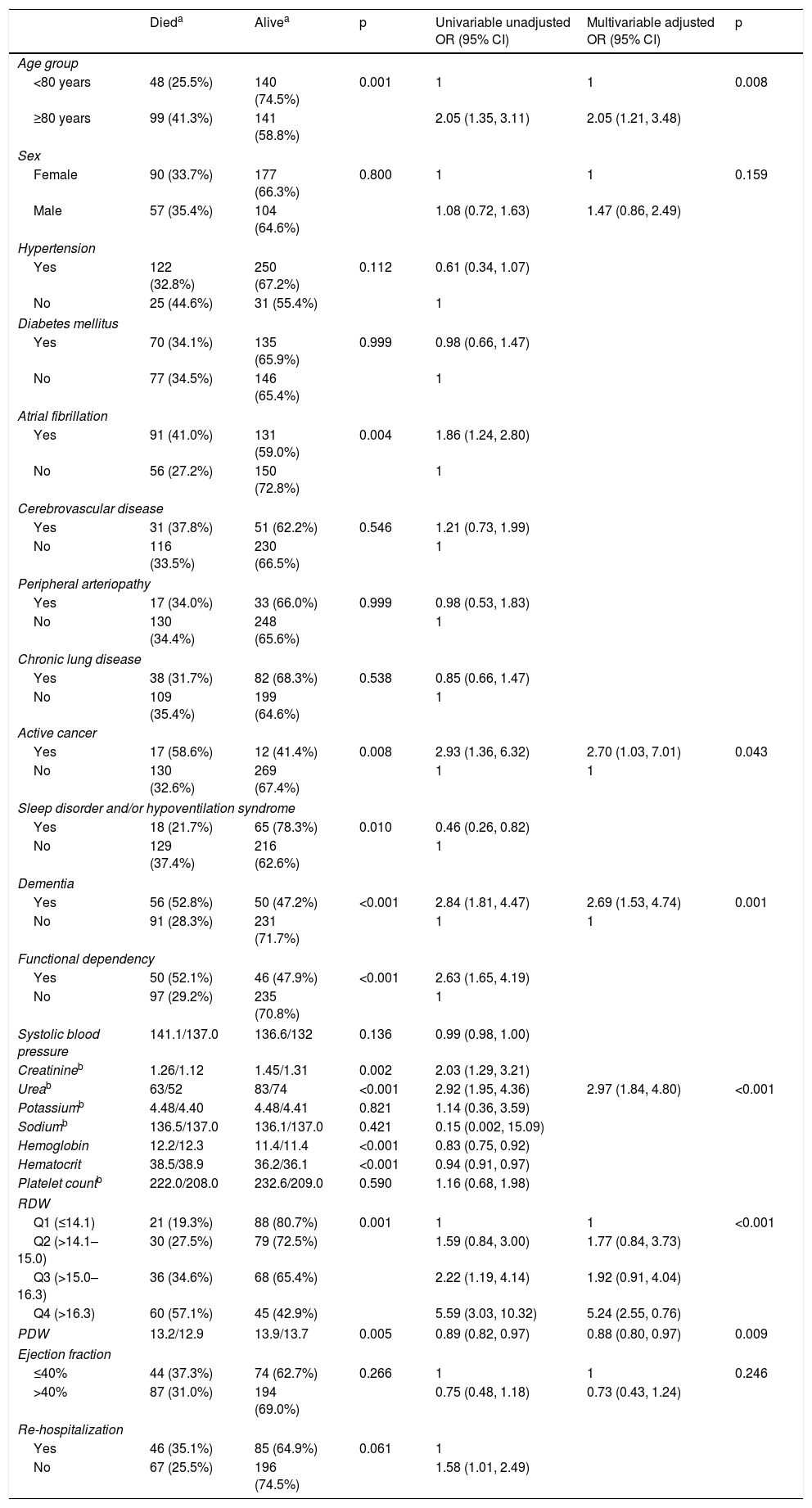
Table 3 summarizes the univariable analysis. Factors significantly associated with higher one-year mortality risk were as follows: age ≥80 years, active cancer, dementia, functional dependency, and AF. Notably, a sleep disorder diagnosis and/or hypoventilation syndrome was associated with a lower one-year mortality risk.
One-year mortality predictors.
| Dieda | Alivea | p | Univariable unadjusted OR (95% CI) | Multivariable adjusted OR (95% CI) | p | |
|---|---|---|---|---|---|---|
| Age group | ||||||
| <80 years | 48 (25.5%) | 140 (74.5%) | 0.001 | 1 | 1 | 0.008 |
| ≥80 years | 99 (41.3%) | 141 (58.8%) | 2.05 (1.35, 3.11) | 2.05 (1.21, 3.48) | ||
| Sex | ||||||
| Female | 90 (33.7%) | 177 (66.3%) | 0.800 | 1 | 1 | 0.159 |
| Male | 57 (35.4%) | 104 (64.6%) | 1.08 (0.72, 1.63) | 1.47 (0.86, 2.49) | ||
| Hypertension | ||||||
| Yes | 122 (32.8%) | 250 (67.2%) | 0.112 | 0.61 (0.34, 1.07) | ||
| No | 25 (44.6%) | 31 (55.4%) | 1 | |||
| Diabetes mellitus | ||||||
| Yes | 70 (34.1%) | 135 (65.9%) | 0.999 | 0.98 (0.66, 1.47) | ||
| No | 77 (34.5%) | 146 (65.4%) | 1 | |||
| Atrial fibrillation | ||||||
| Yes | 91 (41.0%) | 131 (59.0%) | 0.004 | 1.86 (1.24, 2.80) | ||
| No | 56 (27.2%) | 150 (72.8%) | 1 | |||
| Cerebrovascular disease | ||||||
| Yes | 31 (37.8%) | 51 (62.2%) | 0.546 | 1.21 (0.73, 1.99) | ||
| No | 116 (33.5%) | 230 (66.5%) | 1 | |||
| Peripheral arteriopathy | ||||||
| Yes | 17 (34.0%) | 33 (66.0%) | 0.999 | 0.98 (0.53, 1.83) | ||
| No | 130 (34.4%) | 248 (65.6%) | 1 | |||
| Chronic lung disease | ||||||
| Yes | 38 (31.7%) | 82 (68.3%) | 0.538 | 0.85 (0.66, 1.47) | ||
| No | 109 (35.4%) | 199 (64.6%) | 1 | |||
| Active cancer | ||||||
| Yes | 17 (58.6%) | 12 (41.4%) | 0.008 | 2.93 (1.36, 6.32) | 2.70 (1.03, 7.01) | 0.043 |
| No | 130 (32.6%) | 269 (67.4%) | 1 | 1 | ||
| Sleep disorder and/or hypoventilation syndrome | ||||||
| Yes | 18 (21.7%) | 65 (78.3%) | 0.010 | 0.46 (0.26, 0.82) | ||
| No | 129 (37.4%) | 216 (62.6%) | 1 | |||
| Dementia | ||||||
| Yes | 56 (52.8%) | 50 (47.2%) | <0.001 | 2.84 (1.81, 4.47) | 2.69 (1.53, 4.74) | 0.001 |
| No | 91 (28.3%) | 231 (71.7%) | 1 | 1 | ||
| Functional dependency | ||||||
| Yes | 50 (52.1%) | 46 (47.9%) | <0.001 | 2.63 (1.65, 4.19) | ||
| No | 97 (29.2%) | 235 (70.8%) | 1 | |||
| Systolic blood pressure | 141.1/137.0 | 136.6/132 | 0.136 | 0.99 (0.98, 1.00) | ||
| Creatinineb | 1.26/1.12 | 1.45/1.31 | 0.002 | 2.03 (1.29, 3.21) | ||
| Ureab | 63/52 | 83/74 | <0.001 | 2.92 (1.95, 4.36) | 2.97 (1.84, 4.80) | <0.001 |
| Potassiumb | 4.48/4.40 | 4.48/4.41 | 0.821 | 1.14 (0.36, 3.59) | ||
| Sodiumb | 136.5/137.0 | 136.1/137.0 | 0.421 | 0.15 (0.002, 15.09) | ||
| Hemoglobin | 12.2/12.3 | 11.4/11.4 | <0.001 | 0.83 (0.75, 0.92) | ||
| Hematocrit | 38.5/38.9 | 36.2/36.1 | <0.001 | 0.94 (0.91, 0.97) | ||
| Platelet countb | 222.0/208.0 | 232.6/209.0 | 0.590 | 1.16 (0.68, 1.98) | ||
| RDW | ||||||
| Q1 (≤14.1) | 21 (19.3%) | 88 (80.7%) | 0.001 | 1 | 1 | <0.001 |
| Q2 (>14.1–15.0) | 30 (27.5%) | 79 (72.5%) | 1.59 (0.84, 3.00) | 1.77 (0.84, 3.73) | ||
| Q3 (>15.0–16.3) | 36 (34.6%) | 68 (65.4%) | 2.22 (1.19, 4.14) | 1.92 (0.91, 4.04) | ||
| Q4 (>16.3) | 60 (57.1%) | 45 (42.9%) | 5.59 (3.03, 10.32) | 5.24 (2.55, 0.76) | ||
| PDW | 13.2/12.9 | 13.9/13.7 | 0.005 | 0.89 (0.82, 0.97) | 0.88 (0.80, 0.97) | 0.009 |
| Ejection fraction | ||||||
| ≤40% | 44 (37.3%) | 74 (62.7%) | 0.266 | 1 | 1 | 0.246 |
| >40% | 87 (31.0%) | 194 (69.0%) | 0.75 (0.48, 1.18) | 0.73 (0.43, 1.24) | ||
| Re-hospitalization | ||||||
| Yes | 46 (35.1%) | 85 (64.9%) | 0.061 | 1 | ||
| No | 67 (25.5%) | 196 (74.5%) | 1.58 (1.01, 2.49) | |||
CI: confidence interval; OR: odds ratio; PDW: platelet distribution width; Q: quartile; RDW: red cell distribution width.
The biochemical parameters associated with higher mortality risk were as follows: high levels of creatinine, high levels of urea, RDW in the fourth quartile (higher than 16.3%), low levels of hematocrit, and low levels of hemoglobin. High levels of PDW were associated with a lower one-year mortality risk.
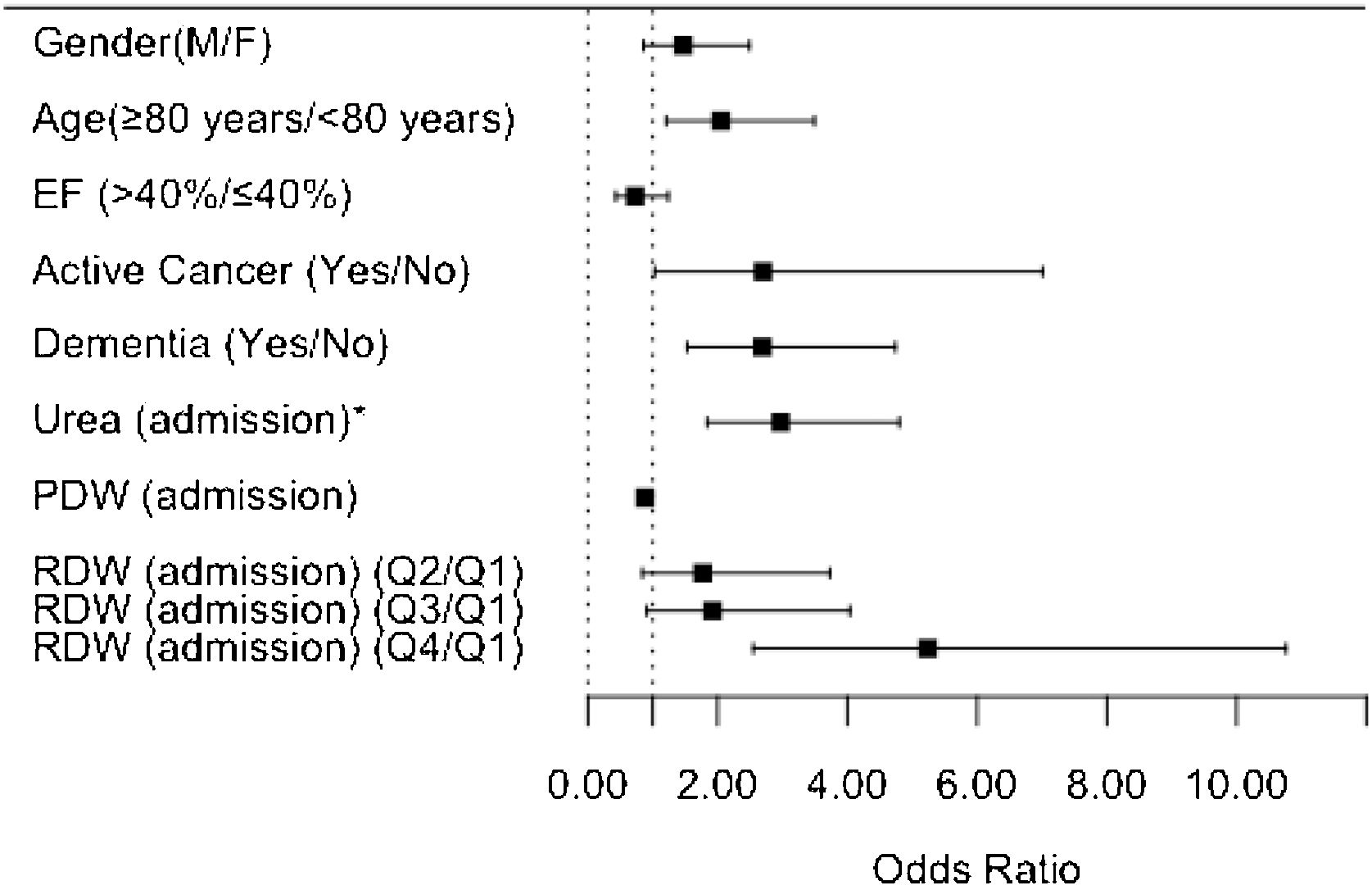
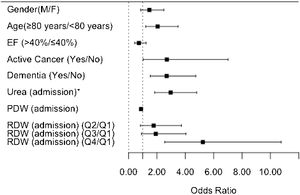
Table 3 also summarizes the multivariable analysis. After adjusting the model for all clinically relevant characteristics (including age, sex, and ejection fraction), the independent predictors of higher one-year mortality risk were as follows: age ≥80 years, active cancer, dementia, high urea levels, RDW in the fourth quartile, and low PDW levels. These findings are also shown in the form of a graph in Figure 1.
DiscussionThis study reports the impact of multiple comorbidities and biochemical parameters on the one-year mortality rate in patients hospitalized for HF in an Internal Medicine Department. After adjusting the multivariable analysis model for all clinically relevant characteristics, the independent predictors of higher one-year mortality risk at admission were: age ≥80 years, active cancer, dementia, high urea levels, high RDW levels, and low PDW levels.
The characteristics of this study population were different from those of clinical studies addressing angiotensin-neprilysin inhibition in stabilized hospitalized patients with HF with reduced ejection fraction (HFrEF).10,11 Our study population was older and predominantly female, with a higher prevalence of hypertension and a lower prevalence of ischemic etiology. This may be explained by the fact that our study included HF patients with and without reduced ejection fraction, which may preclude direct comparison with other studies. As previously discussed, the sex distribution of this study population reflects the fact that elderly patients hospitalized with HF are mainly women.8
The in-hospital and one-year all-cause mortality rates found in this study, 7.9% and 34.3%, respectively, are in accordance with those reported previously in the European database and registry studies,4,5,12 as well as in a Portuguese study.13 This observation confirms the general burden of HF, as expressed by the high one-year mortality rate, ruling out the hypothesis of poor clinical management of HF at local or national levels. Of note, we observed relatively low mortality in the first 30 days after discharge (3.5%). This may be due to the longer hospitalization stays for AHF patients (mean of 10 days in Europe vs. five days in USA), which gives the clinical teams more time to stabilize patients and thereby optimize the therapeutic strategy before hospital discharge.
These findings support the notion that there is still much room for improvement in the standard of care for AHF patients. The current international guidelines for the clinical management of HF need to be further implemented in order to promote better outcomes for HF patients. Under this rationale, risk stratification of HF patients using predictors of increased risk of mortality is a vital tool for ensuring that the most appropriate healthcare plan is implemented for each patient (e.g., frequency of follow-up appointments, assignment to a telemonitoring program, among others).
Other studies have examined the contribution of multiple non-cardiac comorbidities to the mortality of patients with HF.2,14–26 In a pioneer study conducted in 2014, van Dreusen et al. analyzed non-cardiac comorbidities of patients included in the observational Heart Failure Pilot Survey (ESC-HF Pilot) and found that diabetes, chronic kidney disease, and anemia were independent predictors of all-cause mortality risk in a multivariable analysis.2 Since then, several reports, including sub-analyses from clinical studies, have confirmed this association, particularly for chronic kidney disease.19–24 Other predictors for all-cause mortality were also found, such as chronic obstructive pulmonary disease (COPD) and peripheral artery disease.25,26 We could not find any such association in our data, probably due to differences in the baseline characteristics of the population studied. In fact, compared to the pioneer study of Dreusen et al., our study population was older, with more females and fewer patients with HFrEF.2,8
In line with our findings, van Dreusen et al. did not identify sleep apnea as a predictor of mortality. However, in both studies, sleep apnea was not actively investigated; either it was mentioned on the clinical records or self-reported by patients. This may explain the contradiction with studies demonstrating that sleep apnea doubles the mortality risk of HF patients.27 There has also been an increase in the number of studies advocating the importance of sleep-related disorders in patients with cardiovascular disease.28,29 It is of note that van Dreusen et al. did not include dementia and cancer in their analysis.
In the ESC-HF Pilot study, the multivariable analysis found that predictors of one-year mortality in patients hospitalized for HF were: older age, high creatinine, low sodium, low systolic blood pressure, low EF, and other factors not included in our study.4 An Italian observational study reported similar findings.15 However, only older age had a similar result in our analysis. Likewise, we did not find a lower mortality risk in females or patients with higher LVEF, as reported elsewhere,9 despite the higher use of beta-blockers and spironolactone in patients with lower LVEF.8 These differences are probably due to the fact that our patients were all recruited after hospitalization for AHF, unlike those of the other studies, which were primarily outpatients from cardiology centers.
Emerging evidence suggests a relationship between cardiovascular disease and cancer.30,31 HF and cancer are becoming increasingly more prevalent as the population ages, and both conditions are associated with significant mortality and morbidity. Accordingly, our findings that patients admitted for HF, those >80 years, and those with active cancer are more likely to die in the next year, were expected.
Cognitive impairment has a prevalence of 28–58% among HF patients, but little is known about mortality in patients with both diseases.32 Braunstein et al. reported that Alzheimer's disease/dementia is associated with higher mortality risk.14 These observations are in line with the findings of our study since, among the population studied, mortality risk increased with age, HF, and dementia. In fact, as demonstrated by the multivariable analysis, dementia is a robust independent predictor of mortality risk. This finding calls for a dramatic change in the clinical management of dementia patients.
Impairment in renal function is associated with significant morbidity and mortality in patients with chronic HF.2,4,33 We found urea levels to be a strong predictor of one-year mortality. Indeed, blood urea nitrogen (BUN), another way of measuring urea, may increase independently of glomerular filtration rate (GFR) due to enhanced proximal and distal tubular reabsorption under HF neurohormonal activation.33,34 This is especially important in patients with decompensated HF, in whom this mechanism plays a notable role in the clinical manifestations of the disease. Considering these mechanisms, BUN may be a better prognostic indicator than estimated GFR.32 In fact, BUN has been established as a valid marker in HF mortality prediction risk models, both for in-hospital mortality and mortality during the first year after HF hospitalization.35,36 Klein et al. also concluded that high BUN levels at admission are associated with poorer survival rates in HF patients.33
Red cell distribution width is a measure of the variability in the size of circulating erythrocytes and has been linked with a higher mortality risk in both HF patients and the general population.37–39 Our results are consistent with those of previous reports, demonstrating a strong association between high levels of RDW and one-year mortality in patients with AHF, independently of other hematologic parameters, such as hemoglobin and hematocrit levels.37 Remarkably, RDW in the fourth quartile (>16.3%) at admission was found to be a very strong predictor of one-year mortality. Since the evaluation of RDW levels consists of a simple, inexpensive, and widely available test, this finding may have substantial implications for the risk stratification and clinical management of patients with AHF.
Platelet distribution width levels are a widely available platelet activation marker. Very few studies have analyzed the possible effect of PDW on mortality risk in general or specifically in HF patients. The available data suggest that the risk increases with higher values of PDW.40,41 However, we have found a contradictory result in this study, with lower PDW levels independently predicting higher one-year mortality. Thus, PDW may be an important prognostic marker in HF, but its role remains to be clarified in more extensive studies.
Our study has several limitations: (i) it is a retrospective study, (ii) it does not determine the presence and severity of chronic kidney disease, (iii) it does not determine the type, stage, or treatment for oncologic patients, and (iv) it does not examine the use of cardiac devices. Regarding this last point, it is important to stress that it was unlikely that a significant number of this study's patients had implanted such devices, considering their age and that only one-third of them had LVEF ≤40%. Moreover, it is possible that other factors with an impact on mortality have not been included in the analysis. Nevertheless, the findings of this study are sufficiently robust to prompt an improvement in the clinical management of HF patients and contribute to better health outcomes for this population.
ConclusionsThe predictors of one-year mortality in a large cohort of patients hospitalized for HF in an Internal Medicine Department are: age ≥80 years, active cancer, dementia, high values of urea, and RDW at admission. The knowledge of such predictors allows clinicians to tailor both the treatment and follow-up of patients at higher risk of death. These data can be used to establish inexpensive and powerful lab results, such as RDW and urea, as predictors to choose not only the place where the treatment will be held (e.g., cardiac and intensive care units) and the level of monitoring during hospitalization, but also to guide patients to specialized HF clinics and to an earlier post-discharge evaluation. Further studies are needed to corroborate our findings.
FundingNovartis Pharma supported the statistical analysis and the medical writing through an unrestricted grant.
Study oversightL.T. and D.M. performed the statistical analysis, I.M. and R.L.R. prepared the manuscript, and all authors reviewed and approved the final version of the manuscript. All authors vouch for the accuracy and completeness of the data and analysis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Medical writers Duarte Oliveira, Joana Melo, and Carla Gomes (W4Research) collaborated with the authors in the writing of this article.