Transesophageal echocardiography (TEE) has been the standard method for guiding left atrial appendage occlusion (LAAO) procedures. Recently, intracardiac echocardiography (ICE) has emerged as an alternative to TEE due to several advantages, particularly the avoidance of general anesthesia. This analysis aims to assess the safety, feasibility and efficacy of ICE-guided LAAO procedures.
MethodsWe performed a retrospective analysis of ICE-guided LAAO procedures, including a comparison of embolic and bleeding events with the predicted standard scores and a comparison with TEE-guided procedures.
ResultsA total of 88 patients underwent echocardiography-guided percutaneous LAAO (43 patients with TEE and 45 with ICE), mean age 74.9 years, 68.2% male. In the ICE-guided population, the technical success rate was 93% and the major complication rate was 8.8%. During follow-up, yearly stroke and major bleeding rates were 1.4% and 8.4%, respectively, compared to the 4.0% and 8.7% predicted by the CHA2DS2-VASc and HAS-BLED scores. In the TEE versus ICE analysis (similar baseline characteristics), no statistically significant differences were seen regarding technical success (95.3% vs. 93.3%), procedure-related complications (14.0% vs. 8.9%), device thrombus (2.3% vs. 0%), residual minor peridevice leaks (14.0% vs. 24.4%), one-year all-cause mortality (9.3% vs. 4.4%), stroke (9.3% vs. 2.2%) or major bleeding events (9.3% vs. 11.1%).
ConclusionICE-guided LAAO was a safe and effective therapeutic strategy in a high embolic and bleeding risk population, compared to the event rates predicted by the CHA2DS2-VASc and HAS-BLED scores. The ICE-guided procedure compared well to TEE-guided procedures regarding procedure feasibility, safety, and efficacy.
O ecocardiograma transesofágico (ETE) tem vindo a ser utilizado como o método standard para guiar o encerramento de apêndice auricular esquerdo (EAAE). A ecocardiografia intracardíaca (EIC) tem vindo a surgir com método alternativo ao ETE, apresentando várias vantagens, particularmente a evicção de anestesia geral. Esta análise pretende avaliar a segurança, exequibilidade e eficácia de procedimentos de EAAE guiados por EIC.
MétodosAnálise retrospetiva dos pacientes submetidos a EAAE guiado por EIC, incluindo uma comparação das taxas de eventos embólicos e hemorrágicos registados com o previsto pelos scores e comparação com os procedimentos guiados por ETE.
Resultados88 pacientes foram submetidos a EAAE guiado por ecocardiografia (43 por ETE e 45 por EIC), idade média 74,9 anos, 68,2% sexo masculino. Na população guiada por EIC, registou-se sucesso técnico em 93% e complicações major em 8,8%. Durante o período de follow-up, as taxas anuais de acidente vascular cerebral e hemorragias major foram de 1,4% e 8,4% respetivamente, comparados com as taxas anuais de 4,0% e 8,7% previstas pelos scores CHA2DS2-VASc e HAS-BLED. Na análise comparativa entre ETE e EIC (caraterísticas basais da população idênticas), não se registaram diferenças estatisticamente significativas relativamente a sucesso técnico (95,3% versus 93,3%) complicações relacionadas com o procedimento (14,0% versus 8,9%), trombose de dispositivo (2,3% versus 0%), leaks peri-dispositivo (14,0% versus 24,4%), mortalidade global a um ano (9,3% versus 4,4%) ou acidente vascular cerebral (9,3% versus 2,2%) ou hemorragia major a 1 ano (9,3% versus 11,1%).
ConclusãoO EAAE guiado por EIC demonstrou ser uma estratégia terapêutica segura e eficaz numa população com alto risco embólico e hemorrágico, quando comparada com a taxa de eventos prevista pelos scores CHA2DS2-VASc e HAS-BLED. O procedimento guiado por EIC apresentou ainda um bom desempenho quando comparado com o procedimento guiado por ETE, relativamente a exequibilidade, segurança e eficácia.
Atrial fibrillation (AF) is the most common sustained supraventricular arrhythmia in adults, with significant mortality and morbidity, representing an important burden on health care systems.1 Its prevalence in adults is 2–4%,1 increasing with age and in the presence of other comorbidities such as hypertension, diabetes, heart failure (HF), coronary artery disease (CAD), chronic kidney disease (CKD), obesity and obstructive sleep apnea syndrome.2 In general, AF is associated with a five-fold higher risk of stroke.3
Prevention of cardioembolic events in these patients is conventionally carried out using oral anticoagulants (OACs), preferentially direct oral anticoagulants (DOACs), given their efficacy and safety. In a meta-analysis of randomized clinical trials (RCTs), DOACs were associated with a 10% decrease in all-cause mortality, 19% decrease in stroke and 14% decrease in major bleeding (52% decrease in intracranial bleeding, but a 25% increase in gastrointestinal bleeding) compared to vitamin K antagonists (VKAs).4
Left atrial appendage (LAA) occlusion (LAAO) is a safe and effective procedure for prevention of embolic events in patients with AF. It is particularly suitable for patients in whom OACs are contraindicated or who suffer embolic events despite therapeutic anticoagulation.5–7
Transesophageal echocardiography (TEE) has been used as the standard imaging method to guide LAAO, in addition to fluoroscopy, but it usually requires general anesthesia, as well as incurring a small risk of damage to the esophagus. Intracardiac echocardiography (ICE) is a transvascular intracardiac imaging technique used in cardiac structural interventions (including LAAO) that offers the distinct advantage of forgoing general anesthesia.8
ObjectivesThis analysis aims to assess the results of ICE-guided LAAO and to compare ICE- and TEE-guided LAAO in order to assess the safety, feasibility and efficacy of ICE in the guidance of LAAO procedures.
MethodsPatient selectionBetween 2009 and 2020, 88 patients with AF underwent LAAO. Of those, 45 were selected to undergo LAAO with ICE guidance, at the operator's discretion and according to the availability of an anesthesiologist. All patients had a CHA2DS2-VASc score ≥2 and contraindication to OACs (due to high bleeding risk, major or recurrent bleeding history under anticoagulation, bleeding dyscrasia secondary to hematologic disorder or labile international normalized ratio) or inefficacy of OACs as demonstrated by the presence of an LAA thrombus or history of cardioembolic events despite therapeutic oral anticoagulation. Stroke was defined as a focal neurologic defect, with rapidly developing clinical signs and symptoms, lasting for more than 24 hours or leading to death, of probable vascular origin (World Health Organization definition, 2006). Bleeding complications were defined according to the Bleeding Academic Research Consortium criteria (types 2–5).
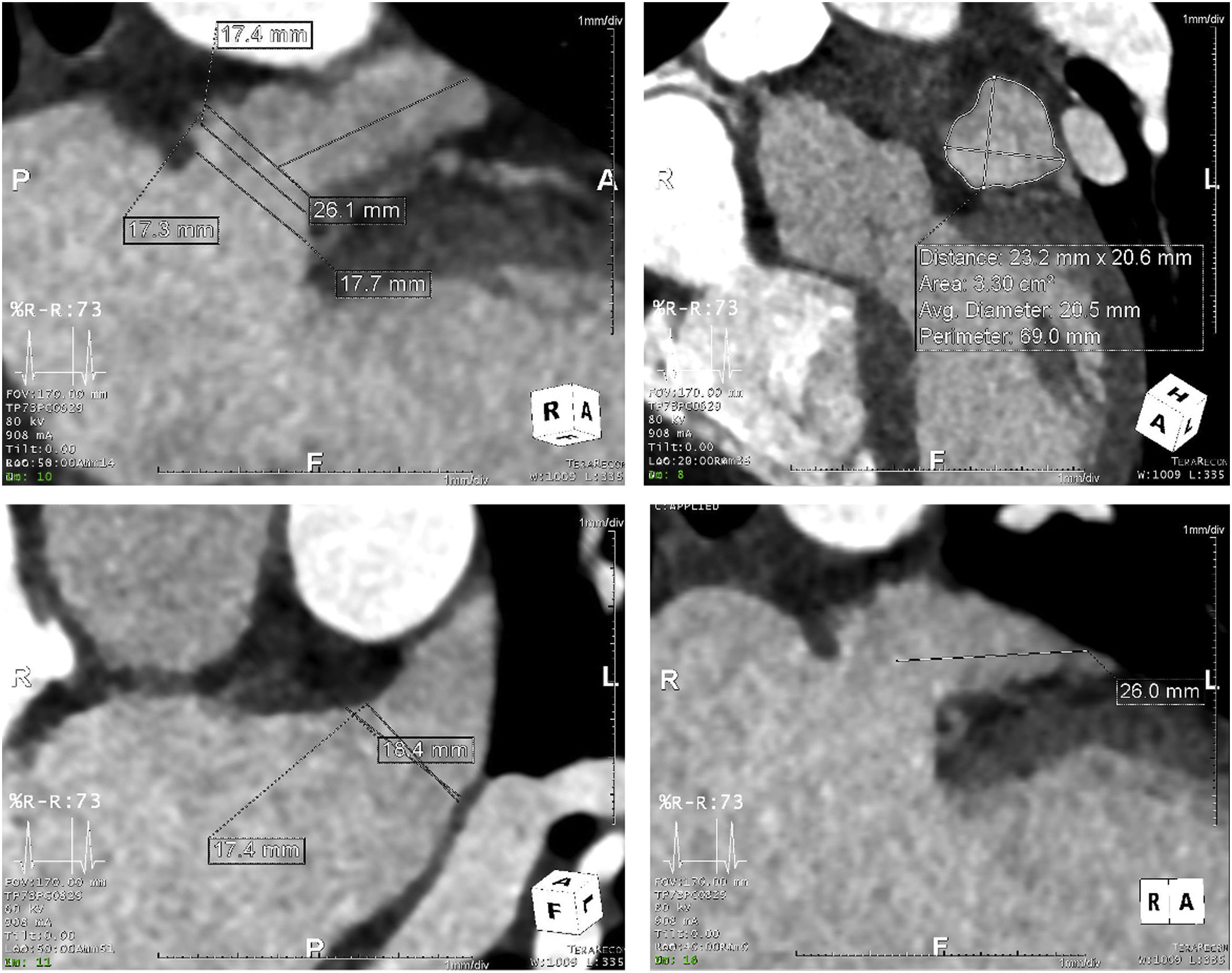
Procedure protocolTo characterize the anatomy and morphology of the LAA and to exclude the presence of thrombus, all patients underwent a cardiac computed angiography (CTA) (example in Figure 1) imaging assessment 48 hours before the procedure or TEE with conscious sedation in the cath lab immediately before LAAO, when CTA imaging was not available.
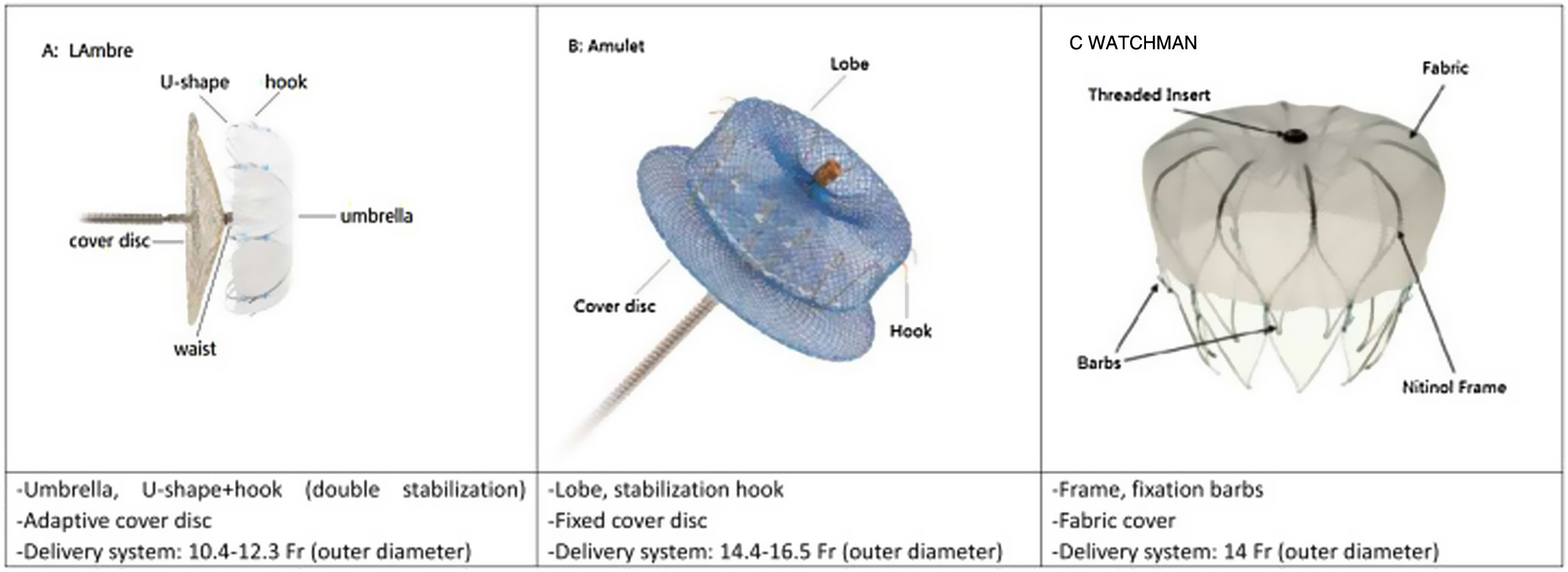
Three types of device (Figure 2) were used for LAAO: Amplatzer Cardiac Plug (ACP) or Amulet (St. Jude Medical), WATCHMAN (Boston Scientific), and LAmbre (Lifetech Scientific). Device sizing was mainly based on the pre-procedural CTA scan or TEE assessment, bearing in mind the angiographic and ICE-acquired dimensions.
All patients underwent LAAO under local anesthesia with or without mild sedation. The procedure was performed using two venous femoral accesses (left femoral for ICE introduction and right femoral for the LAAO device) or two punctures on the same side.
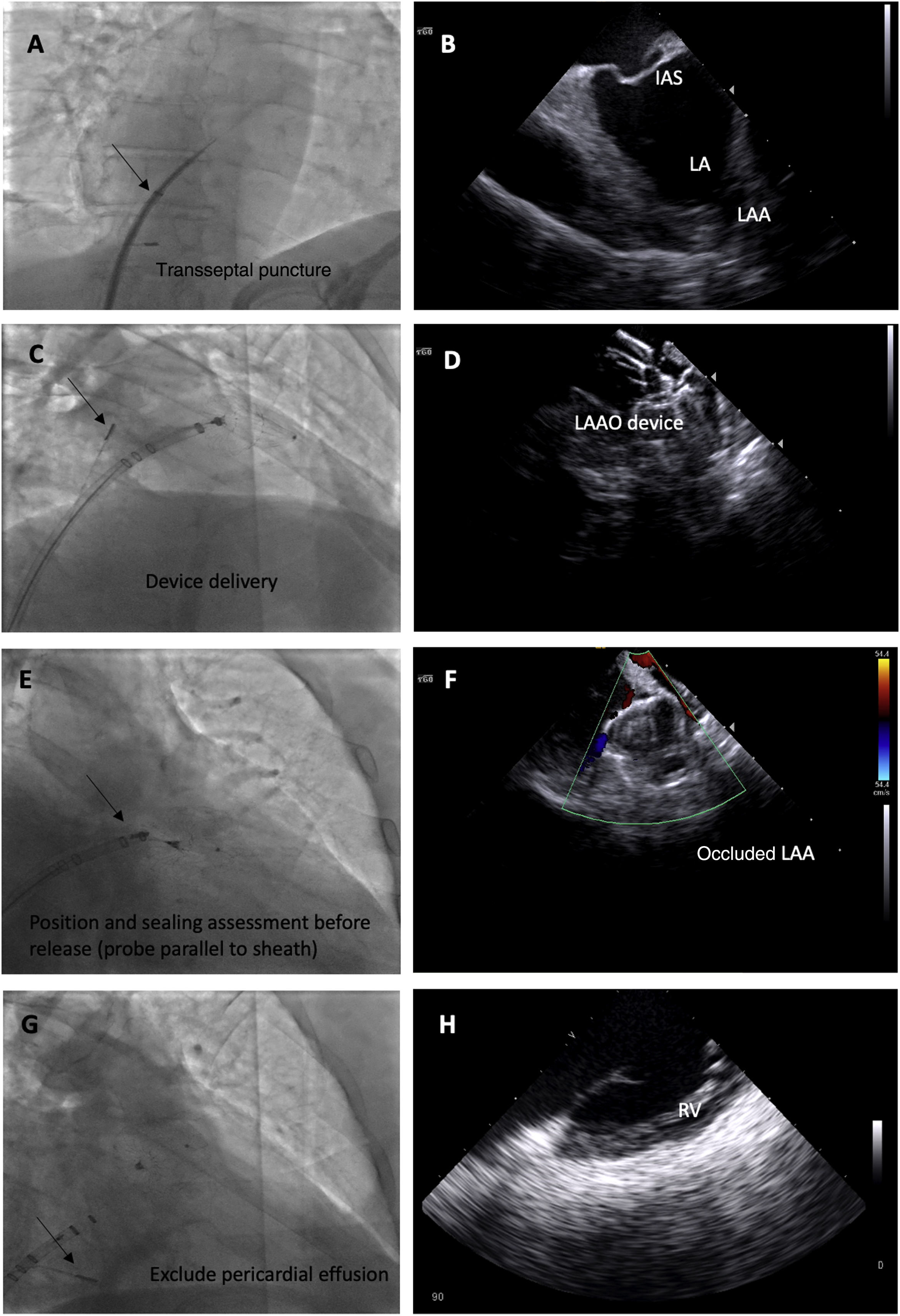
We used the following ICE imaging protocol for LAAO (example images in Figure 3):
- 1.
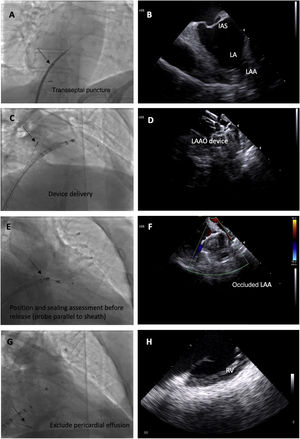
The probe is placed in the mid right atrium with clockwise rotation and posterior tilt to better visualize the fossa ovalis and to guide transseptal puncture in an inferoposterior position. In this view, the LAA is visualized in far field to ensure alignment in the anteroposterior plane and direction of puncture toward the LAA.
- 2.
The transseptal puncture is enlarged with the LAAO device delivery sheath to facilitate the passage of the ICE catheter to the left through the same orifice.
- 3.
The ICE catheter is placed parallel to the LAO with posterior tilt to guide device delivery. This position is used to guide the LAAO device landing zone with the LAA, assess correct positioning and stability before release, rule out significant peridevice leaks and monitor for possible embolization.
- 4.
Finally, after LAAO, the probe is retracted to the right side of the heart and is placed in the right ventricle to exclude pericardial effusion.
Fluoroscopic images of the intracardiac echocardiography probe (black arrows) and its respective echographic images during a left atrial appendage (LAA) occlusion (LAAO) procedure, showing its utility in guiding transseptal puncture (A and B); device deployment (C and D); assessment of device position, significant peridevice leaks using color Doppler and embolization risk (E and F) and exclusion of pericardial effusion (G and H). IAS: interatrial septum.
Post-procedural antithrombotic therapy was decided on an individual basis according to bleeding risk. The most frequently used antithrombotic strategy consisted of six weeks of 2.5 mg apixaban twice daily or dual antiplatelet therapy (DAPT) with 75 mg clopidogrel once daily and 100 mg aspirin once daily. Depending on the clinical course, in the absence of significant complications patients were discharged 24–48 hours after the procedure.
All patients underwent transthoracic echocardiography (TTE) before discharge to rule out device displacement, significant peridevice leak or pericardial effusion, and TEE at six weeks after the procedure to assess device endothelization, the presence of device thrombus, peridevice leaks, or significant atrial septal defects. Patients were followed for a mean of 19±10 months, minimum 12 months. No patients were lost to follow-up.
Statistical analysisBaseline characteristics, procedural data (regarding technical success, complications and procedure-related parameters such as radiation and contrast use), six-week TEE data and follow-up outcomes (stroke, bleeding and all-cause mortality) were noted retrospectively. Continuous variables are presented as means and standard deviation and categorical variables are presented as absolute and relative values (percentage rounded to one decimal place). The recorded stroke and bleeding rates were compared with the rates predicted using the CHA2DS2-VASc and HAS-BLED scores, respectively. Comparisons between TEE and ICE patients were performed using the Student's t test and the chi-square test, and the results presented as odds ratios (OR), 95% confidence intervals and p-values. The statistical analysis was carried out using IBM SPSS version 25.0.
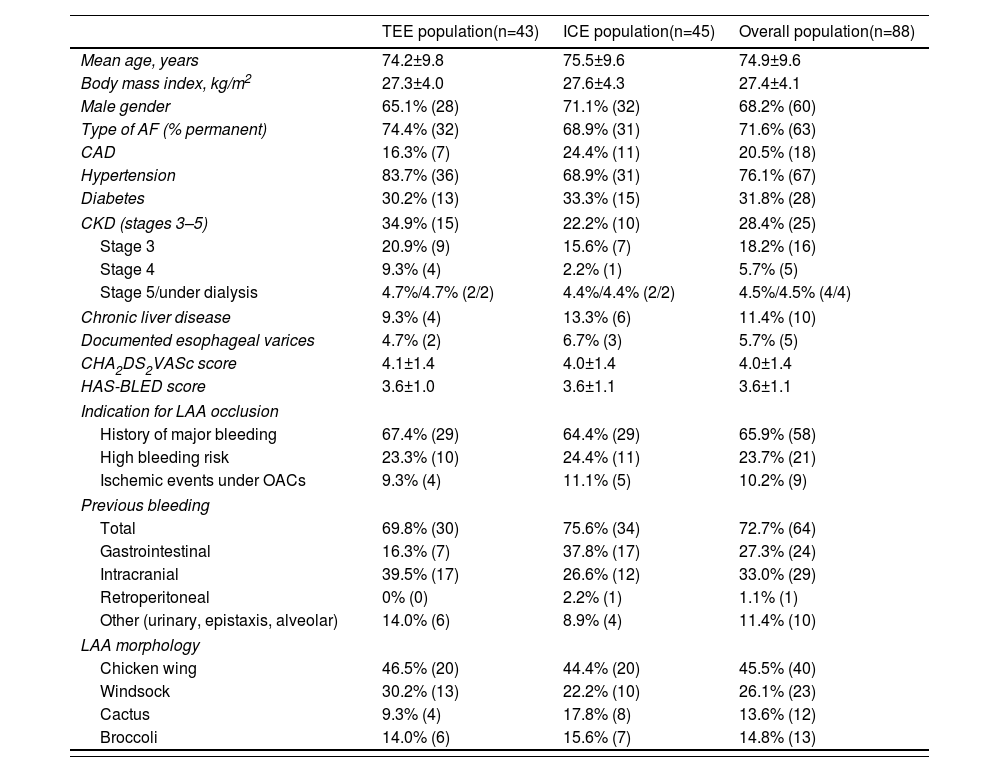
ResultsBaseline characteristics of the study populationA total of 88 patients underwent LAAO (43 patients guided with TEE and 45 guided with ICE), mean age 74.9 years, mainly male (68.2%, n=60), and most with permanent AF (71.6%, n=63). The prevalence of CAD was 20.5%, 31.8% had diabetes, and 28.4% had CKD (stages 3–5 of the Kidney Disease Improving Global Outcomes classification). The prevalence of chronic liver disease was 11.4% and 76.1% had hypertension. Mean CHA2DS2-VASc and HAS-BLED scores were 4.0 and 3.6, respectively, predicting a stroke risk of 4.0% per year and a major bleeding risk of 8.7% per year. The most frequent indication for LAAO was a history of major bleeding (65.9%, n=58). Of note, in some patients the contraindication to oral anticoagulation was established due to the presence of esophageal varices (some with previous upper gastrointestinal bleeding). Baseline characteristics, comorbidities, risk scores and the type of device used for occlusion were similar between the TEE-guided and ICE-guided populations (Table 1).
Detailed baseline characteristics of patients undergoing intracardiac echocardiography-guided left atrial appendage occlusion.
| TEE population(n=43) | ICE population(n=45) | Overall population(n=88) | |
|---|---|---|---|
| Mean age, years | 74.2±9.8 | 75.5±9.6 | 74.9±9.6 |
| Body mass index, kg/m2 | 27.3±4.0 | 27.6±4.3 | 27.4±4.1 |
| Male gender | 65.1% (28) | 71.1% (32) | 68.2% (60) |
| Type of AF (% permanent) | 74.4% (32) | 68.9% (31) | 71.6% (63) |
| CAD | 16.3% (7) | 24.4% (11) | 20.5% (18) |
| Hypertension | 83.7% (36) | 68.9% (31) | 76.1% (67) |
| Diabetes | 30.2% (13) | 33.3% (15) | 31.8% (28) |
| CKD (stages 3–5) | 34.9% (15) | 22.2% (10) | 28.4% (25) |
| Stage 3 | 20.9% (9) | 15.6% (7) | 18.2% (16) |
| Stage 4 | 9.3% (4) | 2.2% (1) | 5.7% (5) |
| Stage 5/under dialysis | 4.7%/4.7% (2/2) | 4.4%/4.4% (2/2) | 4.5%/4.5% (4/4) |
| Chronic liver disease | 9.3% (4) | 13.3% (6) | 11.4% (10) |
| Documented esophageal varices | 4.7% (2) | 6.7% (3) | 5.7% (5) |
| CHA2DS2VASc score | 4.1±1.4 | 4.0±1.4 | 4.0±1.4 |
| HAS-BLED score | 3.6±1.0 | 3.6±1.1 | 3.6±1.1 |
| Indication for LAA occlusion | |||
| History of major bleeding | 67.4% (29) | 64.4% (29) | 65.9% (58) |
| High bleeding risk | 23.3% (10) | 24.4% (11) | 23.7% (21) |
| Ischemic events under OACs | 9.3% (4) | 11.1% (5) | 10.2% (9) |
| Previous bleeding | |||
| Total | 69.8% (30) | 75.6% (34) | 72.7% (64) |
| Gastrointestinal | 16.3% (7) | 37.8% (17) | 27.3% (24) |
| Intracranial | 39.5% (17) | 26.6% (12) | 33.0% (29) |
| Retroperitoneal | 0% (0) | 2.2% (1) | 1.1% (1) |
| Other (urinary, epistaxis, alveolar) | 14.0% (6) | 8.9% (4) | 11.4% (10) |
| LAA morphology | |||
| Chicken wing | 46.5% (20) | 44.4% (20) | 45.5% (40) |
| Windsock | 30.2% (13) | 22.2% (10) | 26.1% (23) |
| Cactus | 9.3% (4) | 17.8% (8) | 13.6% (12) |
| Broccoli | 14.0% (6) | 15.6% (7) | 14.8% (13) |
| Type of occlusion device | TEE populationn=42 | ICE populationn=44 | Overall populationn=86 |
|---|---|---|---|
| WATCHMAN | 57.1% (24) | 61.4% (27) | 59.3% (51) |
| ACP/Amulet | 40.5% (17) | 31.8% (14) | 36.0% (31) |
| LAmbre | 2.3% (1) | 6.8% (3) | 4.7% (4) |
ACP: Amplatzer Cardiac Plug; CAD: coronary artery disease; CKD: chronic kidney disease; ICE: intracardiac echocardiography; LAA: left atrial appendage; OACs: oral anticoagulants; TEE: transesophageal echocardiography.
The LAAO devices were implanted successfully in 94% of patients (n=83), and in 93% of the ICE-guided population (n=42). In the ICE group there was procedural failure in three cases: complete occlusion of the LAA could not be achieved with the available occlusion devices in one patient and device embolization to the left ventricle occurred in the other two, of whom one required urgent heart surgery and the other resulted in cardiac arrest and in-hospital death. In the TEE group, complete LAAO could not be achieved with the available occlusion devices in one patient and in the other, a significant gas embolism occurred, leading to myocardial infarction and death.
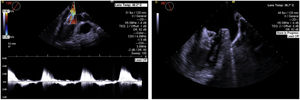
Procedural complicationsThe major complication rate was 8.8% (n=4), consisting in perforation with pericardial effusion requiring pericardiocentesis in 4.4% (n=2) and device embolization (see example in Figure 4) in 4.4% (n=2, one of them leading to cardiac arrest and death), and no major vascular complications occurred. No other procedure-related deaths occurred. The 30-day mortality rate was 2.2% (n=1). Mean length of hospital stay after the procedure was 5.5 days.
Long-term outcomesAll patients were followed for a mean of 19.0 months. During this period, another death (2.2%) occurred from non-cardiac cause, and the rehospitalization rate was 26.7% (n=12), 11.1% (n=5) for cardiac causes. During the same period, one stroke (2.2%) and six significant bleeding events (13.3%) occurred (yearly rates of 1.4% and 8.4%, respectively).
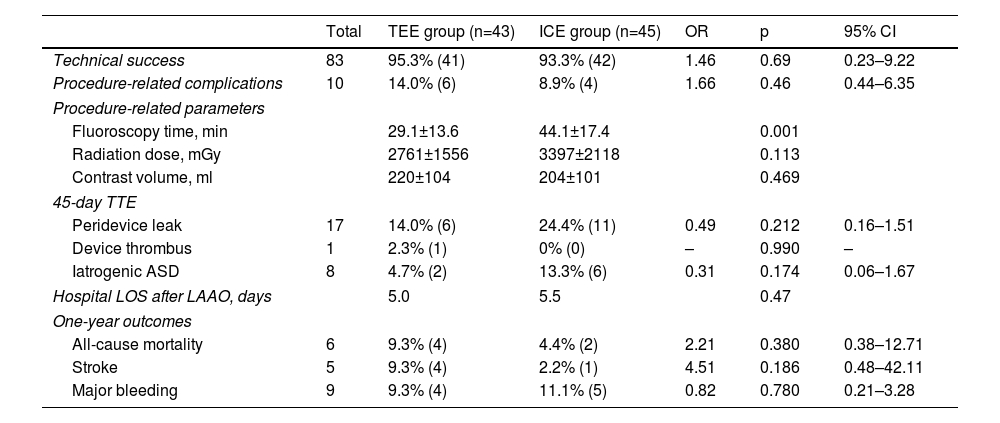
Comparison between transesophageal echocardiography and intracardiac echocardiographyTable 2 summarizes the comparative analysis between ICE- and TEE-guided LAAO. Technical success was achieved in 95.3% (n=41) and 93.3% (n=42) of patients in the TEE and ICE groups, respectively (p=0.69). There was no significant difference in procedure-related complications (major vascular complications, perforation or device embolization) between the TEE and ICE groups (14.0% vs. 8.9%, OR 1.66, p=0.46). Fluoroscopy time was shorter in the TEE group (29.1±13.6 min vs. 44.1±17.4 min, p=0.001), while radiation dose (2761±1555 mGy vs. 3397±2118 mGy, p=0.113) and contrast volume (220.3±104.1 ml, vs. 204.0±100.9 ml, p=0.469) showed no significant differences. TTE at 45 days showed no significant differences between the TEE and ICE groups regarding peridevice leaks (14.0% vs. 24.4%, p=0.212; all of them minor, i.e. less than 5 mm in diameter), device thrombus (2.3% vs. 0%, p=0.99) or persistent iatrogenic atrial septal defects, all mild (4.7% vs. 13.3%, p=0.174). One-year outcomes showed no significant differences regarding stroke (9.3% vs. 4.4%, p=0.186), major bleeding (9.3% vs. 2.2%, p=0.78) or all-cause mortality (9.3% vs. 11.1%, p=0.38) between the TEE and ICE groups, respectively.
Results.
| Total | TEE group (n=43) | ICE group (n=45) | OR | p | 95% CI | |
|---|---|---|---|---|---|---|
| Technical success | 83 | 95.3% (41) | 93.3% (42) | 1.46 | 0.69 | 0.23–9.22 |
| Procedure-related complications | 10 | 14.0% (6) | 8.9% (4) | 1.66 | 0.46 | 0.44–6.35 |
| Procedure-related parameters | ||||||
| Fluoroscopy time, min | 29.1±13.6 | 44.1±17.4 | 0.001 | |||
| Radiation dose, mGy | 2761±1556 | 3397±2118 | 0.113 | |||
| Contrast volume, ml | 220±104 | 204±101 | 0.469 | |||
| 45-day TTE | ||||||
| Peridevice leak | 17 | 14.0% (6) | 24.4% (11) | 0.49 | 0.212 | 0.16–1.51 |
| Device thrombus | 1 | 2.3% (1) | 0% (0) | – | 0.990 | – |
| Iatrogenic ASD | 8 | 4.7% (2) | 13.3% (6) | 0.31 | 0.174 | 0.06–1.67 |
| Hospital LOS after LAAO, days | 5.0 | 5.5 | 0.47 | |||
| One-year outcomes | ||||||
| All-cause mortality | 6 | 9.3% (4) | 4.4% (2) | 2.21 | 0.380 | 0.38–12.71 |
| Stroke | 5 | 9.3% (4) | 2.2% (1) | 4.51 | 0.186 | 0.48–42.11 |
| Major bleeding | 9 | 9.3% (4) | 11.1% (5) | 0.82 | 0.780 | 0.21–3.28 |
ASD: atrial septal defect; CI: confidence interval; ICE: intracardiac echocardiography; LOS: length of stay; OR: odds ratio; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography.
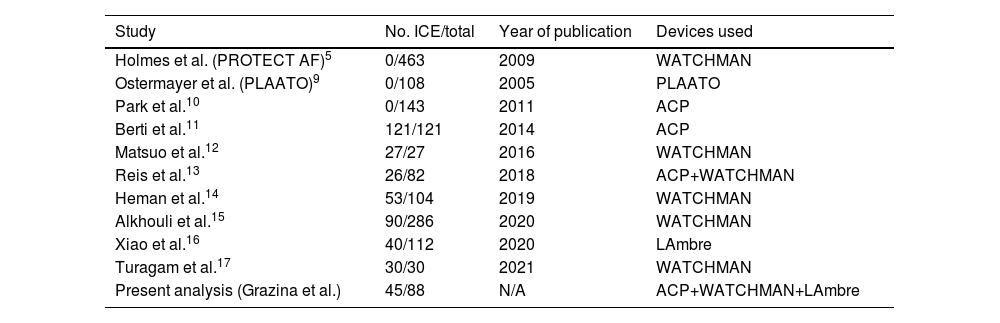
Our study's findings support the use of ICE in the guidance of LAAO, demonstrating its non-inferior feasibility, safety and efficacy compared to TEE. In the first large RCTs comparing LAAO and VKA therapy, all the procedures were TEE-guided.5–7 The advent of ICE guidance of percutaneous structural heart interventions increased interest in its use for guiding LAAO procedures. Placement of the ICE probe in the left atrium (LA) significantly improves LAA image quality compared to placement in the right atrium.8,16 As a result, studies of ICE-guided LAAO in recent years have shown similar results in the feasibility, safety and efficacy of this technique to those of TEE-guided LAAO. Table 3 summarizes some of the more recent LAAO data, with the number of ICE-guided procedures in each study and the type of devices implanted. All showed non-inferior results in ICE-guided LAAO.11–17 Our data confirm the non-inferiority of ICE guidance regarding short-term (success rate and complications) and long-term (stroke, bleeding and all-cause mortality) outcomes.
Summary of left atrial appendage occlusion trials and some recent studies on the use of intracardiac echocardiography to guide the procedure.
| Study | No. ICE/total | Year of publication | Devices used |
|---|---|---|---|
| Holmes et al. (PROTECT AF)5 | 0/463 | 2009 | WATCHMAN |
| Ostermayer et al. (PLAATO)9 | 0/108 | 2005 | PLAATO |
| Park et al.10 | 0/143 | 2011 | ACP |
| Berti et al.11 | 121/121 | 2014 | ACP |
| Matsuo et al.12 | 27/27 | 2016 | WATCHMAN |
| Reis et al.13 | 26/82 | 2018 | ACP+WATCHMAN |
| Heman et al.14 | 53/104 | 2019 | WATCHMAN |
| Alkhouli et al.15 | 90/286 | 2020 | WATCHMAN |
| Xiao et al.16 | 40/112 | 2020 | LAmbre |
| Turagam et al.17 | 30/30 | 2021 | WATCHMAN |
| Present analysis (Grazina et al.) | 45/88 | N/A | ACP+WATCHMAN+LAmbre |
ACP: Amplatzer Cardiac Plug; ICE: intracardiac echocardiography.
Reduced fluoroscopy time, radiation dose and contrast agent dose with ICE are controversial; they have been reported in some studies, but in our analysis the only significant difference was that fluoroscopy time was significantly longer in the ICE group, which may be related to the initial learning curve of ICE use. Although the difference was not significant, a higher rate of residual iatrogenic atrial septal defects was noted at 45 days after the procedure, which can be explained by the passage of the ICE probe to the LA. Nonetheless, most iatrogenic atrial septal defects close spontaneously by 3–12 months, which would explain the high rate at 45 days. Furthermore, in the literature on iatrogenic atrial septal defects after AF ablation or transcatheter mitral procedures, those that persist do not result in increased risk of paradoxical embolism or other clinical manifestations.18,19
The stroke rate in ICE-guided LAAO patients was significantly lower than predicted by the CHA2DS2-VASc score (1.4% vs. 4.0%), while the bleeding rate in ICE-guided LAAO patients was only slightly lower than predicted by the HAS-BLED score (8.4% vs. 8.7%), which can be partly explained by the fact that the ideal post-procedure antithrombotic regimen in patients with high bleeding risk is not well established, which may lead to aggressive regimens, often including full-dose OACs or DAPT. Besides, due to these initial antithrombotic regimens, bleeding events are more likely to occur within a year of the procedure, and thus, considering the short median follow-up, these yearly bleeding rates may overestimate the bleeding risk of subsequent years.
Despite the longer fluoroscopy time, ICE-guided LAAO reduces the need for general anesthesia, thereby requiring fewer human resources and potentially reducing total procedure time (by eliminating induction of and recovery from anesthesia), increasing patient turnover in the procedure room and reducing post-procedural recovery time. Also, in some patients with chronic liver disease and potential or documented esophageal varices, it is desirable to avoid the use of TEE. Possible disadvantages of ICE use include the learning curve of the technique and the need for an additional venous vascular access, although in our experience this did not significantly increase procedure time or vascular complications. We therefore believe that the ICE-guided LAAO approach should be considered a valid alternative approach to LAAO procedures, avoiding general anesthesia, and is to be preferred in frailer patients or in centers with insufficient support from anesthesiology. Although there were no significant differences from TEE-guided LAAO in terms of major complications, we highlight the occurrence of two embolizations, both in the ICE-guided group and with Amulet devices. This motivated us to change the methodology used in the selection of patients and choice of device, prioritizing the WATCHMAN FLX for procedures guided by ICE, as we believe that it is more tolerant of errors in the assessment of LAA dimensions and device stability criteria are easier to check.
Study limitationsThis is a single-center study and the small sample size limits statistical analysis. The data collection and analysis were performed retrospectively. The ICE and TEE groups were not randomized and were thus prone to selection bias. As a result, the likelihood of learning-curve complications may have been lower in the ICE group, thereby limiting direct comparisons between the two groups. The recommended post-procedural antithrombotic regimen in not well established and in our study depended largely on physician criteria. This may have had an impact on stroke and bleeding rates.
ConclusionsICE-guided LAAO was a safe and effective therapeutic strategy in a high embolic and bleeding risk population, compared to the event rates predicted by the CHA2DS2-VASc and HAS-BLED scores. The ICE-guided procedure was shown to be non-inferior to the TEE-guided procedure regarding procedure feasibility, safety and efficacy.
Learning points- 1.
Intracardiac echocardiography is an emerging imaging technique in the guidance of percutaneous structural heart interventions.
- 2.
It has several advantages compared to TEE, the most important being avoidance of general anesthesia, allowing procedures to be performed under local anesthesia only, with or without conscious sedation.
- 3.
Despite the lack of evidence on which to base direct comparisons between these two imaging techniques, our findings further support the use of ICE as a safe and effective alternative to TEE in the context of LAAO procedures.
The authors have no conflicts of interest to declare.