The benefit of manual thrombus aspiration (TA) in the reperfusion of patients with ST-elevation myocardial infarction (STEMI) has been hotly debated. In most series, failure of TA has been largely unreported. Our objectives were to assess the rate, predictors, and impact on cumulative mortality of failed TA during primary percutaneous coronary intervention (PPCI).
MethodsThis was a single-center, retrospective study of consecutive STEMI patients undergoing PPCI with TA. TA was considered ineffective if, before angioplasty, coronary flow was TIMI <2. Independent predictors of TA failure were assessed by logistic regression, and predictors of cumulative mortality were assessed by Cox regression analysis.
ResultsOf 574 patients, TA was used in 417 (72.6%), and was effective in 365 (87.5%) and ineffective in 52 (12.5%). On multivariate analysis, SYNTAX score (OR=1.049, 95% CI: 1.015–1.084, p=0.005) and total ischemic time (OR=1.001, 95% CI: 1.000–1.003, p=0.02) were independent predictors of TA failure. Moderate or severe left ventricular dysfunction (HR=6.256, 95% CI: 1.896–20.644, p=0.003), APPROACH score (HR=1.094, 95% CI: 1.016–1.177, p=0.017), Killip class III/IV (HR=2.953, 95% CI: 1.122–7.770, p=0.028) and creatinine clearance on admission (HR=0.973, 95% CI: 0.953–0.994, p=0.011) were independently related to cumulative mortality at 24±0.82 months.
ConclusionsTotal ischemic time and SYNTAX score were independent predictors of TA failure. However, in medium-term follow-up, ineffective manual TA was not independently related to cumulative mortality.
O benefício da trombectomia aspirativa manual (TbA) na reperfusão do enfarte de miocárdio com elevação de ST (EAMST) tem sido muito debatida. Na maioria das séries, a ineficácia da TbA tem sido pouco evidenciada. Os nossos objetivos visaram conhecer a taxa, os preditores e o impacto na mortalidade cumulativa da TbA ineficaz (TbANE) numa série de doentes submetidos a intervenção coronária percutânea primária (ICPP).
MétodosEstudo retrospetivo, unicêntrico, consecutivo, de doentes com EAMST submetidos a ICPP com TbA. Considerou-se TbANE se após a TbA e antes de prosseguir a angioplastia se se obtivesse fluxo coronário TIMI<2. Identificaram-se preditores independentes de TbANE por regressão logística multivariada. Os preditores de mortalidade cumulativa foram identificados por modelo de Cox.
ResultadosDentre 574 doentes, utilizou-se a TbA em 417 (72,6%), que foi eficaz em 365 (87,5%), ineficaz em 52 (12,5%). Na análise multivariada, o score SYNTAX (OR=1,049, 95% CI: 1,015-1,084, p=0,005) e o tempo isquémico total (OR=1,001, 95% CI: 1,000-1,003, p=0,02) foram os preditores independentes de TbANE. A disfunção ventricular esquerda moderada/severa (HR=6,256, 95% CI: 1,896-20,644, p=0,003), o score APPROACH (HR=1,094, 95% CI: 1,016-1,177, p=0,017), a classe 3-4 de Killip (HR=2,953, 95% CI: 1,122-7,770, p=0,028) e a clearance da creatinina na admissão (HR=0,973, 95% CI: 0,953-0,994, p=0,011), relacionaram-se de forma independente com a mortalidade cumulativa (24±0,82 meses).
ConclusõesO tempo de sintomas e o score SYNTAX foram preditores independentes de TbANE. Contudo, a TbANE não teve impacto independente com a mortalidade cumulativa a médio prazo.
Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease
cardiac magnetic resonance
creatinine clearance
Global Registry of Acute Coronary Events
left ventricular ejection fraction
primary percutaneous coronary intervention
ST-elevation myocardial infarction
Synergy between PCI with TAXUS drug-eluting stent and cardiac surgery
thrombus aspiration
total ischemic time
The benefit of manual thrombus aspiration (TA) as an adjunctive technique in the reperfusion of patients with ST-elevation myocardial infarction (STEMI) has been hotly debated. The advantage of TA during primary percutaneous coronary (PPCI) is that it reduces the risk of distal embolization of thrombotic material during the procedure, thus improving microvascular perfusion and reducing infarct size.1–6 Compared to mechanical thrombus removal, manual TA is simpler to perform and is of equal or superior efficacy.7–9
The introduction of manual TA aroused great interest and it is now widely used in interventional cardiology; it has a class IIa recommendation, level of evidence B, in the European Society of Cardiology guidelines for the management of STEMI.10 Nevertheless, there is considerable variation in use of this technique as adjunctive therapy to reperfusion.11 In a recent survey, manual TA was used in less than 20% of PPCI procedures in the US.12 Although some randomized studies have demonstrated clinical benefit by reducing major cardiovascular events,13–15 subsequently confirmed in meta-analyses,8,16–18 other series, including one large-scale study, have not shown the same clinical efficacy.19–22 However, TA may not be possible in some cases (10% of patients scheduled for TA in the TAPAS study13). The effect of failure of TA in patients in whom it was considered to be indicated has been largely unreported.
The primary objective of this study was to identify the predictors of TA failure in a consecutive series of STEMI patients referred for PPCI, in which TA was systematically used as the first option (bail-out procedures were excluded). The secondary objective was to assess the impact of failed TA on cumulative mortality (cardiac and non-cardiac) in the medium term.
MethodsStudy population and definitionsThis was a single-center retrospective study of consecutive STEMI patients admitted for PPCI between January 2008 and December 2013. Patients undergoing coronary artery bypass grafting were excluded. Clinical, laboratory and procedural data from admission to discharge were collected from hospital records. The vital status of patients during follow-up was assessed by consulting medical records or contact with the attending physician, the patient or relatives. The diagnosis of STEMI was based on clinical criteria, together with the following ECG alterations: ST-segment elevation of ≥0.15 mV in V2-V3 or of ≥0.1 mV in at least two other leads, ST depression of ≥0.15 mm in V2-V3 with positive T wave (posterior infarction), or new-onset complete left bundle branch block. Only patients undergoing manual TA for a documented thrombus in an epicardial coronary artery and TIMI flow 0 or 1 were included in the study.
No reflow was considered present if after angioplasty distal coronary flow was TIMI <2, or TIMI 2–3 with no contrast perfusing the myocardial capillary territory (myocardial perfusion grade 0), or if, after perfusion, stagnation of contrast was observed (myocardial perfusion grade 1), in the absence of spasm, dissection, or epicardial coronary thrombus, that persisted after intracoronary administration of nitroglycerin and adenosine. TA was considered ineffective if, before angioplasty (balloon and/or stent), coronary flow was TIMI <2 and effective with TIMI 2–3.
Total ischemic time (TIT) was defined as the delay between symptom onset (intense, persistent chest pain) and introduction of the guidewire for PPCI. Creatinine clearance (CrCl) was calculated using the Cockcroft-Gault formula.
All patients were risk stratified using the GRACE scores23 for in-hospital and six-month mortality and the TIMI risk score for STEMI.24 The complexity of coronary artery disease was classified using the on-line calculator for the SYNTAX score,25 version 2.11, all lesions with >50% stenosis in vessels of >1.5 mm being included in the analysis. The culprit artery was defined as the one occluded within the previous three months. The area of myocardium at risk was calculated using the modified APPROACH score,26 an angiographic score that estimates the proportion of myocardium that is supplied by a coronary segment based on vessel dominance and caliber. This score is easy to calculate and correlates well with the area at risk as assessed by cardiac magnetic resonance (CMR).27,28 For example, for the proximal anterior descending artery, the area at risk is estimated at 47.75% if there is a large diagonal vessel downstream of the occlusion and at 41.25% if the diagonal is small or nonexistent. The angiographic analyses were performed by two senior interventional cardiologists; in doubtful cases, the scores were arrived at by consensus or taken as the mean of the individual observations.
Left ventricular ejection fraction (LVEF) was assessed by two-dimensional echocardiography (Philips iE33, Eindhoven, The Netherlands) by Simpson's method. LVEF was considered preserved if ≥55%, mildly depressed if ≥45% and <55%, moderately depressed if ≥30% and <45%, and severely depressed if <30%. For the purposes of the statistical analysis, LVEF was classified as preserved or mild dysfunction and moderate or severe dysfunction.
Overall cumulative mortality (cardiac and non-cardiac, in-hospital and during follow-up) was considered a major cardiovascular event. Only patients with a follow-up of ≥12 months after STEMI were included in the analysis.
Procedure and medicationAll patients were medicated with aspirin 300 mg and clopidogrel 600 mg prior to PPCI unless they were already taking these drugs. A 6F or 7F introducer was used. Unfractionated heparin (70 U/kg) was administered during the procedure. Use of glycoprotein IIb/IIIa inhibitors and selection of type of stent and other adjunctive devices were at the operator's discretion. Manual TA was performed using a 6F Export aspiration catheter (Medtronic, Minneapolis, MN, USA) in all cases. Following PPCI, patients were admitted to the coronary care unit. Myocardial necrosis markers (troponin T [TnT], creatine kinase [CK] and CK-MB) were measured at 6, 12, 24 and 48 h after reperfusion. All patients were prescribed aspirin 100 mg/day and clopidogrel 75 mg/day at discharge, together with other medication considered appropriate according to the guidelines.
Statistical analysisThe Kolmogorov-Smirnov test was used to assess the normality of distribution of continuous variables, which were expressed as means ± standard deviation or interquartile range for those with normal and non-normal distribution, respectively. Variables with normal distribution were compared by the Student's t test for independent samples, while those with non-normal distribution were compared by the Mann-Whitney test. Categorical variables were expressed as frequencies and percentages and compared by the chi-square test or by Fisher's exact test. Peak TnT, CK and CK-MB were logarithmically transformed for parametric tests. A logistic regression model was used to identify predictors of TA failure with failed TA as a dependent variable. Unadjusted mortality according to TA efficacy was calculated using the Kaplan-Meier method, the difference being obtained by the log rank test. Cumulative mortality adjusted for confounding variables was assessed using a Cox model. Variables with p<0.1 on univariate analysis were included in the multivariate analysis in order to identify independent predictors. In order to avoid overadjustment, the GRACE and TIMI scores were not included in the regression analysis. A two-tailed p value of p<0.05 was considered significant. The statistical analysis was performed using SPSS version 20 (SPSS, Chicago, IL, US).
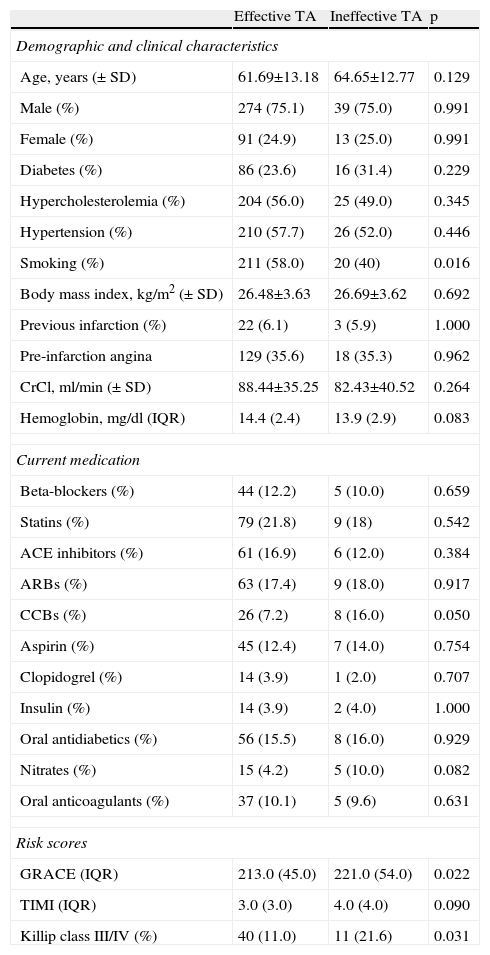
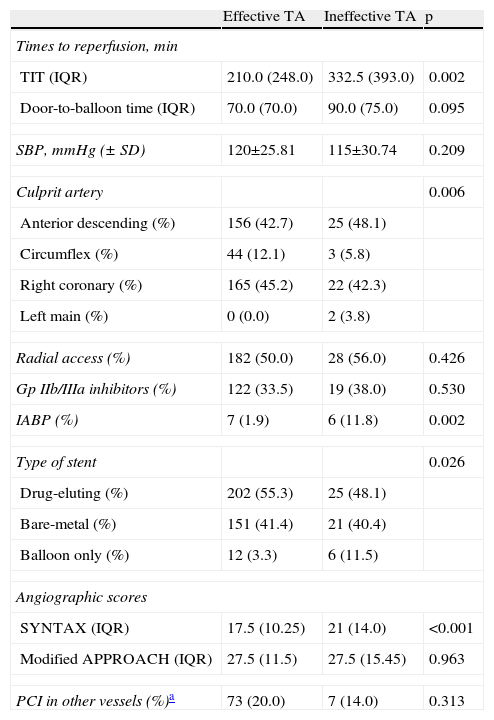
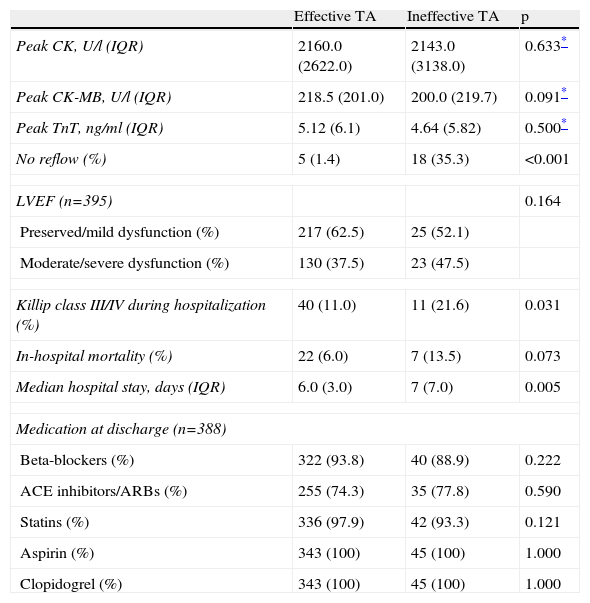
ResultsOf a total of 614 patients with STEMI, 40 were excluded due to lack of data. Of the 574 assessed, TA was used in 417 (72.6%), and was effective in 365 (87.5%) and ineffective in 52 (12.5%). In the latter group, the lesion could not be crossed in four cases (4/52, 7.7%); the operator proceeded to balloon dilatation of the lesions, but TA was attempted again in only one of these patients, which was also unsuccessful. Tables 1 and 2 summarize the clinical and procedure-related characteristics, respectively, of the study population, according to the efficacy of TA. TA failure was significantly associated with non-smokers, higher GRACE and SYNTAX scores and Killip class at admission, and longer TIT. With regard to the procedure, the circumflex was less frequently the culprit artery, and an intra-aortic balloon pump was more frequently used in those with ineffective TA. There was a tendency for a higher rate of previous therapy with nitrates and calcium channel blockers in the ineffective TA group, and lower hemoglobin at admission. In terms of in-hospital outcome (Table 3), the rate of no reflow was higher in those with failed TA. No significant differences were found in peak CK, CK-MB or TnT, or in LVEF at discharge, although it should be noted that enzyme peaks could not be measured in the first 24 h in 21 patients (3.7%), mainly due to death soon after the procedure (n=19 [90.5%]). Of these 21 patients, TA was used in 12 (57.1%), and was effective in seven (58.3%) and ineffective in five (41.7%). Hospital stay was longer in the ineffective TA group. Mortality was also higher (13.5% vs. 6%, p=0.073) in this group, but without statistical significance.
Demographic and clinical characteristics, medication and risk scores.
| Effective TA | Ineffective TA | p | |
| Demographic and clinical characteristics | |||
| Age, years (± SD) | 61.69±13.18 | 64.65±12.77 | 0.129 |
| Male (%) | 274 (75.1) | 39 (75.0) | 0.991 |
| Female (%) | 91 (24.9) | 13 (25.0) | 0.991 |
| Diabetes (%) | 86 (23.6) | 16 (31.4) | 0.229 |
| Hypercholesterolemia (%) | 204 (56.0) | 25 (49.0) | 0.345 |
| Hypertension (%) | 210 (57.7) | 26 (52.0) | 0.446 |
| Smoking (%) | 211 (58.0) | 20 (40) | 0.016 |
| Body mass index, kg/m2 (± SD) | 26.48±3.63 | 26.69±3.62 | 0.692 |
| Previous infarction (%) | 22 (6.1) | 3 (5.9) | 1.000 |
| Pre-infarction angina | 129 (35.6) | 18 (35.3) | 0.962 |
| CrCl, ml/min (± SD) | 88.44±35.25 | 82.43±40.52 | 0.264 |
| Hemoglobin, mg/dl (IQR) | 14.4 (2.4) | 13.9 (2.9) | 0.083 |
| Current medication | |||
| Beta-blockers (%) | 44 (12.2) | 5 (10.0) | 0.659 |
| Statins (%) | 79 (21.8) | 9 (18) | 0.542 |
| ACE inhibitors (%) | 61 (16.9) | 6 (12.0) | 0.384 |
| ARBs (%) | 63 (17.4) | 9 (18.0) | 0.917 |
| CCBs (%) | 26 (7.2) | 8 (16.0) | 0.050 |
| Aspirin (%) | 45 (12.4) | 7 (14.0) | 0.754 |
| Clopidogrel (%) | 14 (3.9) | 1 (2.0) | 0.707 |
| Insulin (%) | 14 (3.9) | 2 (4.0) | 1.000 |
| Oral antidiabetics (%) | 56 (15.5) | 8 (16.0) | 0.929 |
| Nitrates (%) | 15 (4.2) | 5 (10.0) | 0.082 |
| Oral anticoagulants (%) | 37 (10.1) | 5 (9.6) | 0.631 |
| Risk scores | |||
| GRACE (IQR) | 213.0 (45.0) | 221.0 (54.0) | 0.022 |
| TIMI (IQR) | 3.0 (3.0) | 4.0 (4.0) | 0.090 |
| Killip class III/IV (%) | 40 (11.0) | 11 (21.6) | 0.031 |
ACE: angiotensin-converting enzyme; ARBs: angiotensin II receptor blockers; CCBs: calcium channel blockers; CrCl: creatinine clearance; IQR: interquartile range; SD: standard deviation; TA: thrombus aspiration.
Procedure-related variables.
| Effective TA | Ineffective TA | p | |
| Times to reperfusion, min | |||
| TIT (IQR) | 210.0 (248.0) | 332.5 (393.0) | 0.002 |
| Door-to-balloon time (IQR) | 70.0 (70.0) | 90.0 (75.0) | 0.095 |
| SBP, mmHg (± SD) | 120±25.81 | 115±30.74 | 0.209 |
| Culprit artery | 0.006 | ||
| Anterior descending (%) | 156 (42.7) | 25 (48.1) | |
| Circumflex (%) | 44 (12.1) | 3 (5.8) | |
| Right coronary (%) | 165 (45.2) | 22 (42.3) | |
| Left main (%) | 0 (0.0) | 2 (3.8) | |
| Radial access (%) | 182 (50.0) | 28 (56.0) | 0.426 |
| Gp IIb/IIIa inhibitors (%) | 122 (33.5) | 19 (38.0) | 0.530 |
| IABP (%) | 7 (1.9) | 6 (11.8) | 0.002 |
| Type of stent | 0.026 | ||
| Drug-eluting (%) | 202 (55.3) | 25 (48.1) | |
| Bare-metal (%) | 151 (41.4) | 21 (40.4) | |
| Balloon only (%) | 12 (3.3) | 6 (11.5) | |
| Angiographic scores | |||
| SYNTAX (IQR) | 17.5 (10.25) | 21 (14.0) | <0.001 |
| Modified APPROACH (IQR) | 27.5 (11.5) | 27.5 (15.45) | 0.963 |
| PCI in other vessels (%)a | 73 (20.0) | 7 (14.0) | 0.313 |
Percutaneous coronary intervention in non-culprit vessels during the same hospitalization. Gp: glycoprotein; IABP: intra-aortic balloon pump; IQR: interquartile range; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; SD: standard deviation; TA: thrombus aspiration; TIT: total ischemia time.
In-hospital outcome.
| Effective TA | Ineffective TA | p | |
| Peak CK, U/l (IQR) | 2160.0 (2622.0) | 2143.0 (3138.0) | 0.633* |
| Peak CK-MB, U/l (IQR) | 218.5 (201.0) | 200.0 (219.7) | 0.091* |
| Peak TnT, ng/ml (IQR) | 5.12 (6.1) | 4.64 (5.82) | 0.500* |
| No reflow (%) | 5 (1.4) | 18 (35.3) | <0.001 |
| LVEF (n=395) | 0.164 | ||
| Preserved/mild dysfunction (%) | 217 (62.5) | 25 (52.1) | |
| Moderate/severe dysfunction (%) | 130 (37.5) | 23 (47.5) | |
| Killip class III/IV during hospitalization (%) | 40 (11.0) | 11 (21.6) | 0.031 |
| In-hospital mortality (%) | 22 (6.0) | 7 (13.5) | 0.073 |
| Median hospital stay, days (IQR) | 6.0 (3.0) | 7 (7.0) | 0.005 |
| Medication at discharge (n=388) | |||
| Beta-blockers (%) | 322 (93.8) | 40 (88.9) | 0.222 |
| ACE inhibitors/ARBs (%) | 255 (74.3) | 35 (77.8) | 0.590 |
| Statins (%) | 336 (97.9) | 42 (93.3) | 0.121 |
| Aspirin (%) | 343 (100) | 45 (100) | 1.000 |
| Clopidogrel (%) | 343 (100) | 45 (100) | 1.000 |
p value calculated by the t test for independent samples after logarithmic transformation of CK, CK-MB and TnT. ACE: angiotensin-converting enzyme; ARBs: angiotensin receptor blockers; CK: creatine kinase; IQR: interquartile range; LVEF: left ventricular ejection fraction; TA: thrombus aspiration; TnT: troponin T.
On univariate analysis, age, SYNTAX score, current smoking, admission hemoglobin, previous therapy with nitrates or calcium channel blockers, TIT and Killip class III/IV at admission correlated with ineffective TA. In multivariate analysis, only SYNTAX score (odds ratio [OR]: 1.049, 95% confidence interval [CI]: 1.015–1.084, p=0.005) and TIT (OR: 1.001, 95% CI: 1.000–1.003, p=0.02) were independent predictors.
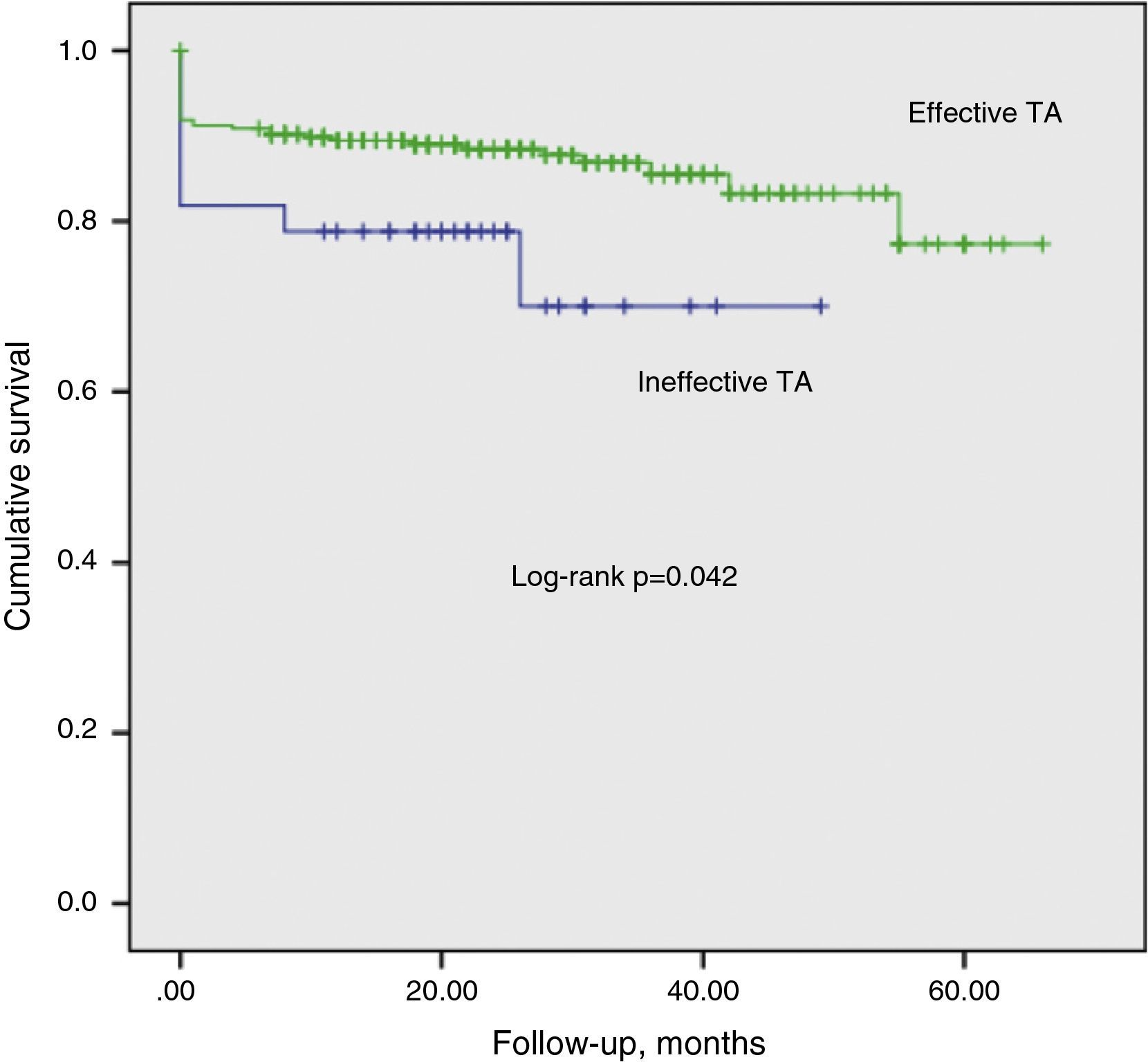
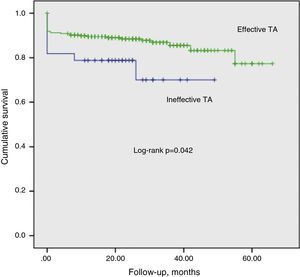
We identified 331 patients with at least 12 months of follow-up after STEMI. It was not possible to ascertain vital status in two of these (0.6%), and so 329 patients were included in the analysis of medium-term mortality (mean follow-up: 24±0.82 months, cumulative mortality rate: 13.9%). Unadjusted cumulative mortality was higher in the ineffective TA group (Figure 1). After adjustment for covariables with p<0.1 on univariate analysis (gender, age, TA efficacy, APPROACH and SYNTAX scores, current smoking, TIT, CrCl, target vessel, intra-aortic balloon pump, hemoglobin, systolic blood pressure, Killip class III/IV at admission and LVEF at discharge), LVEF showing moderate or severe dysfunction (hazard ratio [HR]: 6.256, 95% CI: 1.896–20.644, p=0.003), APPROACH score (HR: 1.094, 95% CI: 1.016–1.177, p=0.017), and Killip class III/IV (HR: 2.953, 95% CI: 1.122–7.770, p=0.028) showed a positive correlation with mortality, while higher CrCl values had a protective effect (HR: 0.973, 95% CI: 0.953–0.994, p=0.011).
DiscussionIn this series, TA was scheduled in the majority of patients (72.6%), and was effective in most cases (87.5%). It is plausible that the more organized the thrombus, the less effective TA will be, and it is thus not surprising that the longer the delay between symptom onset and reperfusion, the greater the likelihood of TA failure. The correlation between TIT and thrombus organization as a predictor of adverse events has been reported in previous studies.29–32 However, the relationship between SYNTAX score and TA failure is unknown. The technique is more likely to be unsuccessful in patients with unfavorable coronary anatomy such as tortuosity or calcification, or with a large amount of plaque that is at risk of distal embolization, resulting in impaired microvascular perfusion. A correlation between SYNTAX score and risk of no reflow has been demonstrated in some series of patients undergoing PPCI.33
Excluding patients in whom TA was not performed, for example because the vessel involved was of small caliber or there was insufficient thrombotic material to justify TA, systematic use of the technique might be expected to reduce infarct size when effective. However, this assumption was not confirmed in our series. Although peak enzyme levels can only estimate infarct size, they correlate well with infarct size as assessed by CMR.34,35 In studies using CMR to assess the area of necrosis, patients undergoing TA did not show reduced infarct size,22,36 even in cases with less microvascular obstruction.20
With regard to mortality, no significant differences in clinical endpoints would be expected in view of the small sample size. However, the trend observed in in-hospital mortality could be more marked in larger series. Unsurprisingly, in the patient subgroup assessed for cumulative mortality, LVEF showing moderate to severe dysfunction and Killip class III/IV were strong independent predictors of medium-term mortality. These two parameters,37–39 together with the area of myocardium at risk,40 have been reported as markers of adverse prognosis. An interesting possibility is that TA may only have a significant impact in patients with large areas of at-risk myocardium,41 but specially designed prospective studies are needed to confirm this hypothesis. Renal failure has often been cited as an independent cause of mortality in STEMI patients,42,43 and so it is to be expected that higher CrCl values are associated with a protective effect.
The debate concerning the benefits of TA in reperfusion is far from consensus.44 The conflicting results of studies published to date may be due to sample sizes being too small to assess clinical endpoints and to different methods of evaluating coronary microvascular perfusion. At the same time, it appears that not all patients benefit to the same extent from TA, reflecting the heterogeneity of STEMI patients. Only large-scale studies or meta-analyses will provide solid evidence on the most important clinical endpoints, and so our results concerning mortality should be interpreted with caution. It is known that patients with no reflow have increased long-term mortality.45 The significant relationship found between no reflow and ineffective TA may be reflected in increased cumulative mortality in larger series, which would enable this association to be studied in more detail. Overall, the results of this observational study are similar to the latest series comparing TA with conventional PPCI. With the exception of a lower rate of no reflow, effective TA did not result in an unequivocal benefit compared to ineffective TA.
Study limitationsLimitations include the fact that this was a single-center retrospective study and with too small a sample size to assess the impact of TA efficacy on medium-term mortality. The rate of TA failure was low (<15%), which may have affected the results.
ConclusionsIn our series, TA was ineffective in 12.5% of cases, and TIT and SYNTAX score were independent predictors of TA failure. On multivariate analysis, ineffective TA was not related to larger infarct size or increased medium-term mortality.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Luz A, Rodrigues P, Sousa MJ, et al. A trombectomia aspirativa na reperfusão do enfarte agudo de miocárdio: preditores e impacto clínico da sua ineficácia. Rev Port Cardiol. 2014;33:753–760.