Increased activation of the sympathetic nervous system plays a central role in the pathophysiology of hypertension (HTN). Catheter-based renal denervation (RDN) was recently developed for the treatment of resistant HTN.
AimTo assess the safety and efficacy of RDN for blood pressure (BP) reduction at six months in patients with resistant HTN.
MethodsIn this prospective registry of patients with essential resistant HTN who underwent RDN between July 2011 and May 2013, the efficacy of RDN was defined as ≥10 mmHg reduction in office systolic blood pressure (SBP) six months after the intervention.
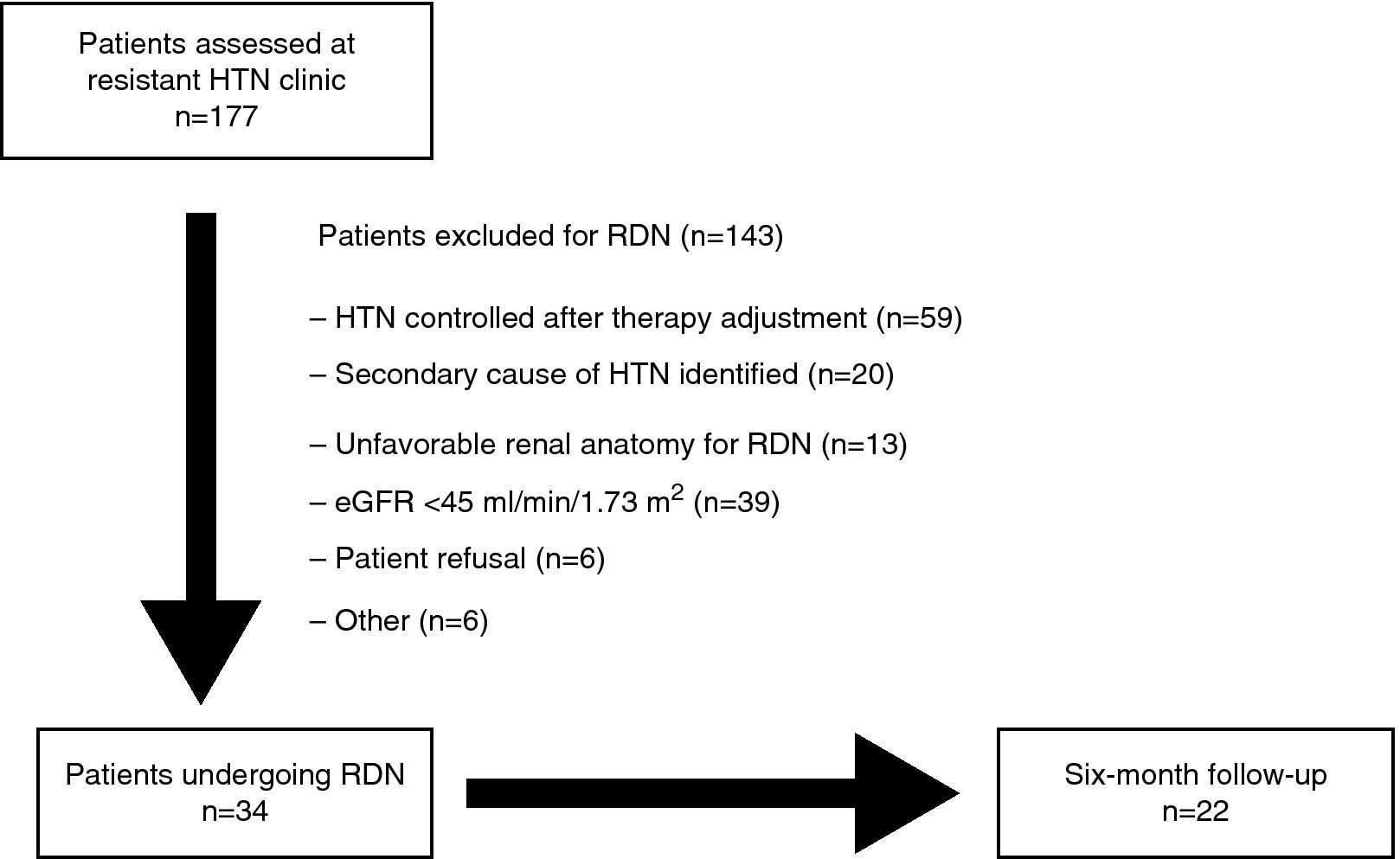
ResultsIn a resistant HTN outpatient clinic, 177 consecutive patients were evaluated, of whom 34 underwent RDN (age 62.7±7.6 years; 50.0% male). There were no vascular complications, either at the access site or in the renal arteries. Of the 22 patients with complete six-month follow-up, the response rate was 81.8% (n=18). The mean office SBP reduction was 22 mmHg (174±23 vs. 152±22 mmHg; p<0.001) and 9 mmHg in diastolic BP (89±16 vs. 80±11 mmHg; p=0.006). The number of antihypertensive drugs (5.5±1.0 vs. 4.6±1.1; p=0.010) and pharmacological classes (5.4±0.7 vs. 4.6±1.1; p=0.009) also decreased significantly. Of the 24-hour ambulatory BP monitoring and echocardiographic parameters analyzed, there were significant reductions in diastolic load (45±29 vs. 27±26%; p=0.049) and in left ventricular mass index (174±56 vs. 158±60 g/m2; p=0.014).
ConclusionIn this cohort of patients with resistant HTN, RDN was safe and effective, with a significant BP reduction at six-month follow-up.
O aumento da atividade do sistema nervoso simpático desempenha um papel preponderante na fisiopatologia da hipertensão arterial (HTA). Recentemente foi desenvolvida uma técnica de intervenção percutânea – a desnervação renal (DNR) – para o tratamento da HTA resistente.
ObjetivoAvaliar a segurança imediata e a eficácia da DNR aos seis meses na redução da pressão arterial em doentes com HTA resistente.
MétodosRegisto prospetivo de doentes com HTA essencial resistente submetidos a DNR entre julho de 2011 e maio de 2013. A eficácia da DNR foi definida pela redução ≥10 mmHg da pressão arterial sistólica (PAS), avaliada na consulta dos seis meses de seguimento.
ResultadosNuma consulta de HTA resistente avaliaram-se 177 doentes consecutivos, dos quais 34 (idade 62,7±7,6 anos; 50,0% homens) efetuaram DNR. Não ocorreram complicações vasculares, nomeadamente no acesso ou nas artérias renais. Nos 22 doentes com seguimento completo aos seis meses, a taxa de respondedores foi 81,8% (n=18). A PAS na consulta diminuiu em média 22 mmHg (174±23 versus 152±22 mmHg; p<0,001) e a diastólica 9 mmHg (89±16 versus 80±11 mmHg; p=0,006). O número de fármacos anti-hipertensores (5,5±1,0 versus 4,6±1,1; p=0,010) e de classes farmacológicas (5,4±0,7 versus 4,6±1,1; p=0,009) também diminuíram significativamente. Dos parâmetros da monitorização ambulatória da pressão arterial de 24 h e ecocardiográficos analisados, a percentagem de cargas diastólicas (45±29 versus 27±26%; p=0,049) e o índice de massa ventricular esquerda (174±56 versus 158±60 g/m2; p=0,014) diminuíram significativamente.
ConclusãoNa população estudada de doentes com HTA resistente submetidos a DNR, esta foi uma intervenção segura e eficaz na redução da pressão arterial aos seis meses de seguimento.
Hypertension (HTN) is one of the main independent risk factors for global mortality.1 Its high prevalence and increasing incidence, including among young adults, are a major public health concern.2
Despite the many approved and recommended therapeutic options, the rate of control of HTN is far from ideal.3 This was demonstrated by the PAP study on the prevalence, awareness, treatment and control of HTN in Portugal,4 which showed not only a high prevalence of HTN in individuals aged 18 and over (42.1%) but also a low rate of control (11.2%). Although various factors contribute to poor control, in a significant number of cases HTN is resistant to drug therapy and it is therefore essential to identify such patients given their high risk of cardiovascular events.5–7 The limitations of current drug therapies probably reflect the complex pathophysiological mechanisms involved in the development and persistence of HTN.8,9 Chronic activation of the sympathetic nervous system is an important mechanism in resistant HTN, and so a new interventional technique – renal denervation (RDN) – has been developed, consisting of endovascular application of radiofrequency energy in the renal arteries to modulate renal sympathetic activity.10,11
The safety and efficacy of RDN were first documented in the Symplicity HTN-111 and Symplicity HTN-2 trials,12 and there is evidence that similar levels of blood pressure (BP) reduction are maintained in the medium term.13,14 We recently published our initial experience with this technique to treat patients with resistant HTN.15
The aim of this study was to assess the safety and efficacy of RDN for BP reduction at six months in patients with resistant HTN.
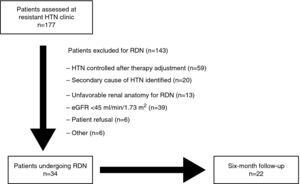
MethodsStudy design and populationIn this prospective registry of 177 consecutive patients evaluated in the resistant HTN outpatient clinic of a tertiary center between July 2011 and May 2013, resistant HTN was defined as office BP of ≥140/90 mmHg despite therapy with at least three antihypertensive drugs (including a diuretic) at maximum tolerated doses.16 Possible secondary causes of HTN were excluded in all patients. Patients were selected for RDN in joint meetings between the cardiologists and nephrologists responsible for patient assessment in the HTN clinic. The procedures were approved by the hospital's ethics committee and patients’ informed consent was obtained. The study design is summarized in Figure 1. The criteria used in selecting patients for RDN were recently published by de Araújo Gonçalves et al.15
After clinical and laboratory assessment in accordance with the protocol, 34 patients were selected for RDN, of whom 22 completed six-month follow-up. The final analysis assessed the immediate safety of the procedure in all patients and its efficacy in the group with complete six-month follow-up.
Clinical assessment and diagnostic examsRenal artery angiography was performed in all patients to assess anatomical suitability for RDN, and in 73.5% (n=25) of those considered eligible, noninvasive computed tomography angiography was performed prior to RDN. The anatomical criteria were renal artery diameter ≥4 mm and absence of significant tortuosity or >50% stenosis. Demographic variables, clinical history and anthropometric data were recorded. Baseline assessment prior to RDN included systolic (SBP) and diastolic blood pressure (DBP) at the last consultation, transthoracic echocardiography, 24-hour ambulatory BP monitoring (ABPM) and laboratory tests. Antihypertensive medication was also recorded, both the number of drugs and pharmacological classes, divided into the following categories: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, direct renin inhibitors, aldosterone antagonists, diuretics, beta-blockers, calcium-channel blockers and alpha-blockers.
Renal denervation procedureThe procedure was performed via femoral access in all cases except one in which the left radial artery was used. After gaining vascular access, abdominal aortography and selective renal artery angiography were performed. Radiofrequency energy was applied in both renal arteries using the following systems: Symplicity® (Medtronic, USA) in 26 patients, EnligHTN® (St. Jude Medical, USA) in six, and OneShot® (Covidien, USA) in two. The device is connected to a radiofrequency generator that automatically programs and controls impedance, temperature and duration of the application, independently of the operator, on the basis of the manufacturer's protocols for each type of device. The Symplicity® system performs 4–6 applications lasting 120 s each in both renal arteries, beginning in the most distal segment of the vessel, at intervals of around 5 mm and in different quadrants of the arterial wall.11 EnligHTN® is a multi-electrode system that provides multiple applications without the need to maneuver the device; the procedure also begins with the most distal electrode with four sequential applications lasting 90 s each, the ideal being two series of four applications in each artery.17 The more recently approved OneShot® system uses a guidewire and a single irrigated balloon-mounted spiral electrode that applies energy for 120 s.18 All procedures were performed under sedation with anesthesia support (propofol and remifentanil in weight-adjusted doses) and anticoagulation with unfractionated heparin for a minimum activated clotting time of 250 s. In all cases of femoral access, the access site was closed using an Angio-Seal® (St. Jude Medical, USA). There were no complications at the access site or in the renal arteries following RDN; there was one case of renal artery spasm and stenosis on final angiographic assessment, in a procedure performed on an accessory renal artery with a diameter at the lower recommended limit (4 mm).
Follow-upTo assess the efficacy of RDN at six months, we used the definition of responder used in validation studies of the technique: reduction in office SBP of ≥10 mmHg at follow-up. Office DBP, number of antihypertensive drugs and pharmacological classes, and 24-hour ABPM values were also assessed at follow-up, as well as the following echocardiographic parameters: left ventricular mass index (LVMI), left ventricular ejection fraction (LVEF), left atrial volume index, E/A ratio (E and A representing maximum early and late mitral flow velocities, respectively, by pulsed Doppler), E wave deceleration time, and E/e′ ratio (e′ representing mitral annular early diastolic velocity by tissue Doppler).
The immediate safety of RDN was assessed on the basis of complications related to the vascular access site (hematoma or pseudoaneurysm) or to selective renal artery catheterization or radiofrequency application (spasm, stenosis, dissection, thrombosis or perforation).
Statistical analysisThe statistical analysis was performed using Statistical Package for Social Sciences® for Windows, version 19.0 (SPSS, Inc., Chicago, IL). Categorical variables were expressed as frequencies (percentages in brackets) and compared using Fisher's exact test. Continuous variables were expressed as means ± standard deviation and compared using the Student's t test when appropriate. Results with p<0.05 were considered statistically significant.
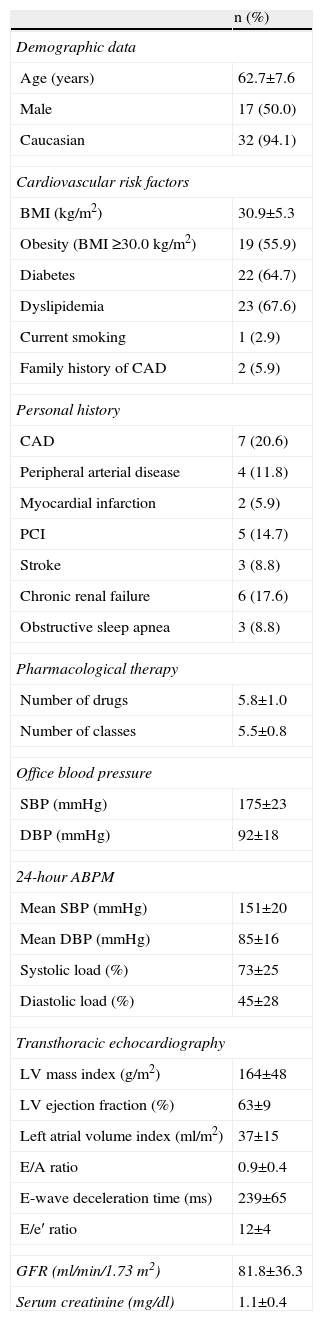
ResultsBaseline characteristics of patientsThe baseline characteristics of patients undergoing RDN are shown in Table 1. Their mean age was 62.7±7.6 years, 50% were male (n=17) and most (94.1%, n=32) were Caucasian. Cardiovascular risk factors included obesity in 55.9% (mean body mass index 30.9±5.3 kg/m2), type 2 diabetes in 64.7%, dyslipidemia in 67.6%, current smoking in 2.9%, and family history of premature coronary artery disease (CAD) in 5.9%. Personal history included vascular disease in any territory in 32.4% (n=11) – peripheral arterial disease in 11.8% (n=4), cerebrovascular disease in 8.8% (n=3), and CAD in 20.6% (5.9% with previous myocardial infarction and 14.7% with percutaneous coronary intervention). Three patients (8.8%) had concomitant obstructive sleep apnea and their elevated BP levels persisted despite home noninvasive ventilatory support. Mean estimated glomerular filtration rate (eGFR) was 81.8±36.3 ml/min/1.73 m2; 17.6% had chronic renal failure, defined as eGFR <60 ml/min/1.73 m2. Mean serum creatinine was 1.1±0.4 mg/dl.
Baseline characteristics of the study population (n=34).
| n (%) | |
| Demographic data | |
| Age (years) | 62.7±7.6 |
| Male | 17 (50.0) |
| Caucasian | 32 (94.1) |
| Cardiovascular risk factors | |
| BMI (kg/m2) | 30.9±5.3 |
| Obesity (BMI ≥30.0 kg/m2) | 19 (55.9) |
| Diabetes | 22 (64.7) |
| Dyslipidemia | 23 (67.6) |
| Current smoking | 1 (2.9) |
| Family history of CAD | 2 (5.9) |
| Personal history | |
| CAD | 7 (20.6) |
| Peripheral arterial disease | 4 (11.8) |
| Myocardial infarction | 2 (5.9) |
| PCI | 5 (14.7) |
| Stroke | 3 (8.8) |
| Chronic renal failure | 6 (17.6) |
| Obstructive sleep apnea | 3 (8.8) |
| Pharmacological therapy | |
| Number of drugs | 5.8±1.0 |
| Number of classes | 5.5±0.8 |
| Office blood pressure | |
| SBP (mmHg) | 175±23 |
| DBP (mmHg) | 92±18 |
| 24-hour ABPM | |
| Mean SBP (mmHg) | 151±20 |
| Mean DBP (mmHg) | 85±16 |
| Systolic load (%) | 73±25 |
| Diastolic load (%) | 45±28 |
| Transthoracic echocardiography | |
| LV mass index (g/m2) | 164±48 |
| LV ejection fraction (%) | 63±9 |
| Left atrial volume index (ml/m2) | 37±15 |
| E/A ratio | 0.9±0.4 |
| E-wave deceleration time (ms) | 239±65 |
| E/e′ ratio | 12±4 |
| GFR (ml/min/1.73 m2) | 81.8±36.3 |
| Serum creatinine (mg/dl) | 1.1±0.4 |
ABPM: ambulatory blood pressure monitoring; BMI: body mass index; CAD: coronary artery disease; DBP: diastolic blood pressure; GFR: glomerular filtration rate; LV: left ventricular; PCI: percutaneous coronary intervention; SBP: systolic blood pressure.
At the last consultation prior to RDN, mean SBP and DBP were 175±23 mmHg and 92±18 mmHg, respectively, and mean heart rate was 71±18 bpm, while 24-hour ABPM showed the following mean values: SBP 151±20 mmHg, DBP 85±16 mmHg, mean arterial pressure 107±14 mmHg, pulse pressure 68±16 mmHg, systolic load 73±25%, diastolic load 45±28% and heart rate 69±12 bpm, with absence of circadian rhythm in 57.1% of patients. Transthoracic echocardiography revealed left ventricular (LV) hypertrophy in most patients (90.9%), with mean LVMI of 164±48 g/m2. Mean LVEF was 66±9%, and only four patients presented reduced LVEF (<55% by Simpson's biplane method). Mean left atrial volume index was 37±15 g/m2, E/A ratio 0.9±0.4, E-wave deceleration time 239±65 ms and E/e′ ratio 12±4. On average, patients were medicated with 5.8±1.0 antihypertensive drugs, from 5.5±0.8 pharmacological classes. The most commonly prescribed drug classes were calcium channel blockers, used in 97.1% (n=33), diuretics in 88.2% (n=30) and beta-blockers in 82.4% (n=28). Both aldosterone antagonists and alpha-blockers were prescribed in 70.6% (n=24), angiotensin receptor blockers in 61.8% (n=21), angiotensin-converting enzyme inhibitors in 52.9% (n=18) and renin inhibitors in 14.7% (n=5).
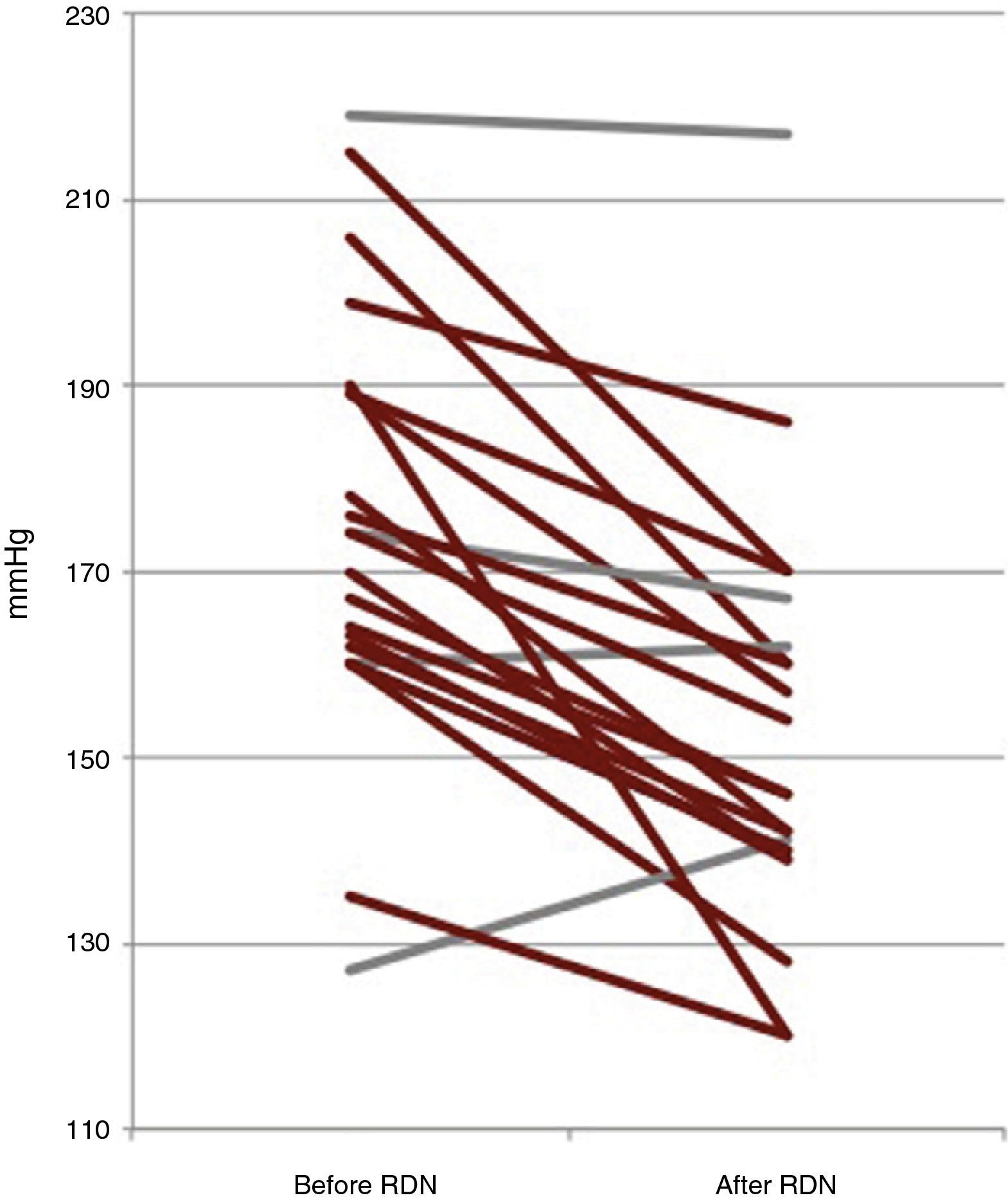
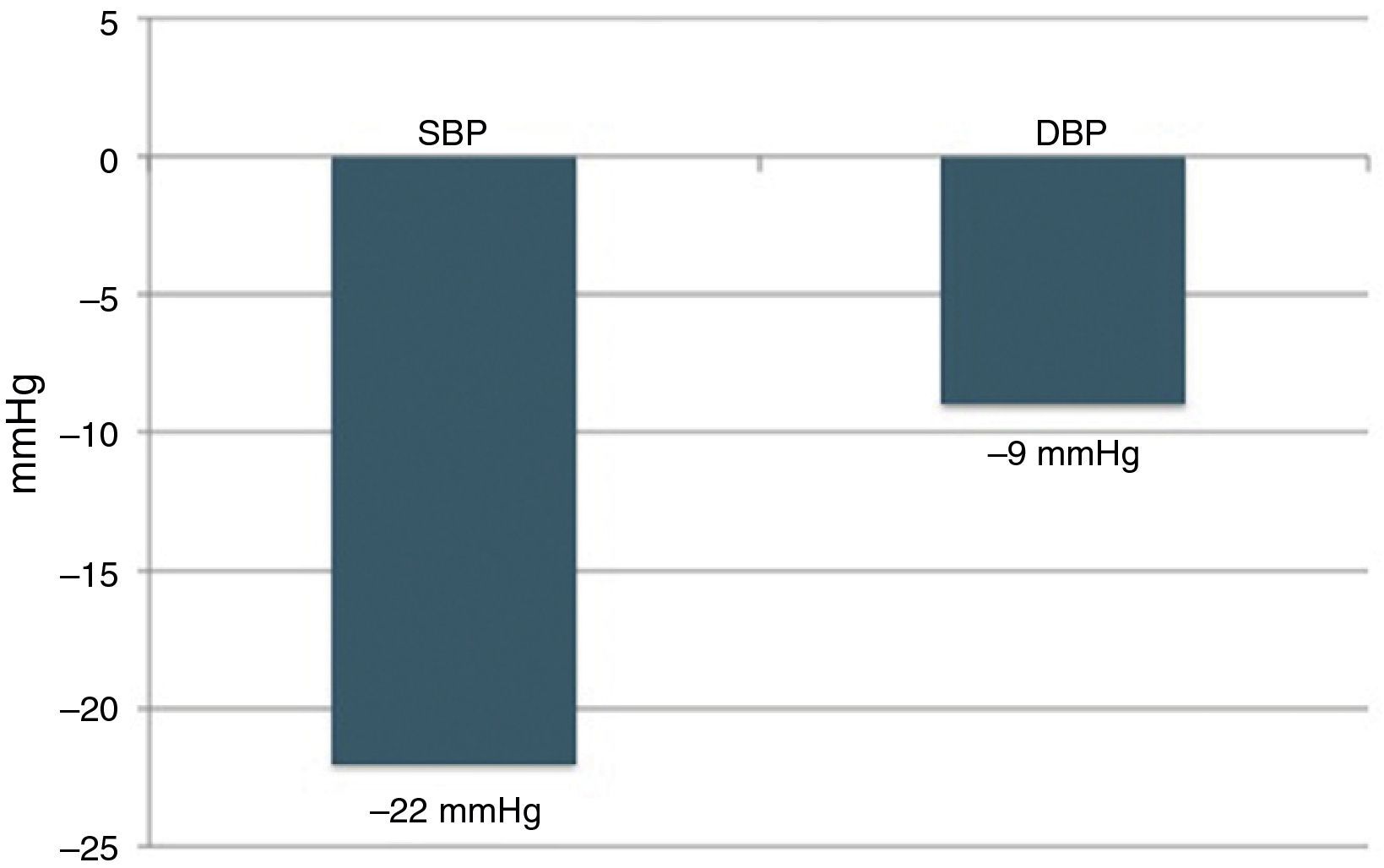
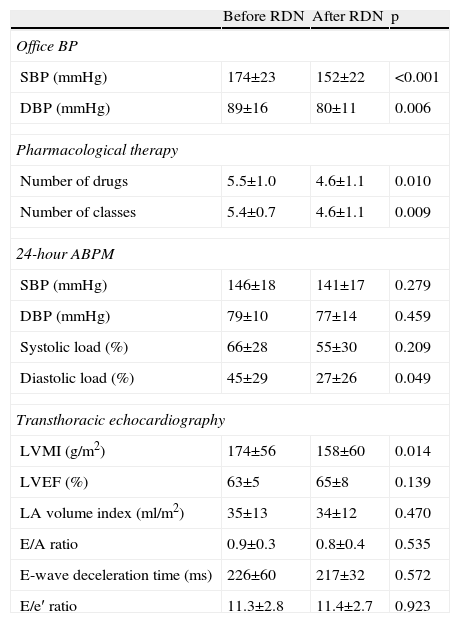
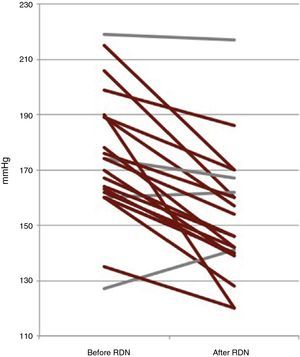
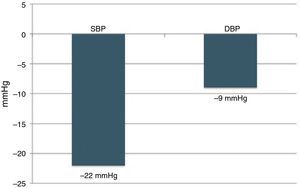
Six-month follow-upOf the 22 patients with complete six-month follow-up, 18 (81.8%) were considered responders (Figure 2). Of the four non-responders, only one had higher BP after RDN than the baseline value, while the other three showed reductions of less than 10 mmHg. Mean office SBP decreased by 22 mmHg, a statistically significant reduction (174±23 vs. 152±22 mmHg, p<0.001), and mean DBP also fell significantly, by 9 mmHg (89±16 vs. 80±11 mmHg, p=0.006) (Figure 3). Other parameters that changed significantly six months after RDN were diastolic load on 24-hour ABPM (45±29% vs. 27±26%, p=0.049) and LVMI (174±56 vs. 158±60 g/m2, p=0.014). The echocardiographic parameters used to assess systolic and diastolic function did not change significantly, nor did serum creatinine (1.0±0.3 vs. 1.0±0.4 mg/dl, p=0.344) (Table 2). The number of antihypertensive drugs (5.5±1.0 vs. 4.6±1.1, p=0.010) and pharmacological classes (5.4±0.7 vs. 4.6±1.1, p=0.009) also decreased significantly after RDN. It was not possible to compare the different RDN systems since patients treated by the OneShot® (n=2) and EnligHTN® (n=6) systems had not completed the six-month follow-up period. However, their inclusion in the study did enable the baseline characteristics of patients selected for RDN to be described, and the immediate safety of the procedure to be assessed.
Office blood pressure, pharmacological therapy, 24-hour ambulatory blood pressure monitoring and echocardiographic parameters before and six months after renal denervation (n=22).
| Before RDN | After RDN | p | |
| Office BP | |||
| SBP (mmHg) | 174±23 | 152±22 | <0.001 |
| DBP (mmHg) | 89±16 | 80±11 | 0.006 |
| Pharmacological therapy | |||
| Number of drugs | 5.5±1.0 | 4.6±1.1 | 0.010 |
| Number of classes | 5.4±0.7 | 4.6±1.1 | 0.009 |
| 24-hour ABPM | |||
| SBP (mmHg) | 146±18 | 141±17 | 0.279 |
| DBP (mmHg) | 79±10 | 77±14 | 0.459 |
| Systolic load (%) | 66±28 | 55±30 | 0.209 |
| Diastolic load (%) | 45±29 | 27±26 | 0.049 |
| Transthoracic echocardiography | |||
| LVMI (g/m2) | 174±56 | 158±60 | 0.014 |
| LVEF (%) | 63±5 | 65±8 | 0.139 |
| LA volume index (ml/m2) | 35±13 | 34±12 | 0.470 |
| E/A ratio | 0.9±0.3 | 0.8±0.4 | 0.535 |
| E-wave deceleration time (ms) | 226±60 | 217±32 | 0.572 |
| E/e′ ratio | 11.3±2.8 | 11.4±2.7 | 0.923 |
ABPM: ambulatory blood pressure monitoring; BP: blood pressure, DBP: diastolic blood pressure; LA: left atrial; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; RDN: renal denervation; SBP: systolic blood pressure.
RDN proved to be safe in this group of patients with resistant HTN, with no serious complications. There were no access site complications such as pseudoaneurysm; one patient was the first published case of RDN via radial access.19 There were no cases of renal artery dissection, thrombosis or rupture, and only one case of spasm and stenosis, observed at the end of the procedure performed on an accessory renal artery with a diameter at the lower recommended limit.
The safety of RDN was first demonstrated in 2009 by the Symplicity HTN-1 study,11 which enabled the technique to be introduced into clinical practice. Nevertheless, the studies in the literature involved small numbers of patients, and follow-up periods are still too short to draw definitive conclusions on the technique's safety in the medium to long term.
As well as demonstrating its safety, our study showed RDN to be effective in reducing office BP at six-month follow-up, with a mean reduction in SBP of 22 mmHg; there was a reduction in SBP of at least 10 mmHg in 82% of cases, as well as a significant fall in mean DBP (9 mmHg). Its impact on office BP also meant that the number of antihypertensive drugs prescribed decreased significantly.
The growing number of hypertensive patients and the morbidity and mortality associated with poor BP control, due in part to resistant HTN, point to the need for alternative therapeutic approaches. It is estimated that around seven million deaths and 64 million disability-adjusted life years each year can be attributed to poorly controlled HTN.20 Literature reviews indicate that around 15% of hypertensive patients may have resistant HTN,5,6 which occurs more frequently in men, those aged >55 years, blacks, and those with diabetes, obesity or chronic end-stage renal failure.5,6,8,9
Management of patients with resistant HTN is complex; it is essential to rule out secondary causes of HTN, optimize drug therapy and exclude white coat or other pseudo-resistant forms of HTN prior to applying advanced techniques such as RDN. The low percentage of patients in a resistant HTN outpatient clinic who were considered suitable for RDN in the present study demonstrates the complexity of patient selection for this technique, the ratio of the total number assessed to those considered eligible being approximately 5:1 (19.2%).
Pharmacological therapy in HTN is mainly based on drugs that act on the renin-angiotensin-aldosterone system, the sympathetic nervous system being considered of secondary importance. However, the role of sympathetic modulation in HTN was demonstrated more than half a century ago. An association has been shown between sympathetic nervous system activation and different forms and stages of HTN, including the earliest.21–24 In addition, the effect of sympathectomy in reducing BP has also been demonstrated, although this technique was abandoned due to procedure-related complications and the subsequent development of antihypertensive drugs.25–27 Recent advances in miniaturized devices for radiofrequency ablation have made percutaneous sympathetic denervation possible and have renewed interest in intervention in the relationship between sympathetic activity and HTN.
Our results are similar to those of previous studies demonstrating the efficacy of RDN in patients with resistant HTN. The Symplicity HTN-2 study,12 the first randomized trial to show BP reduction at six-month follow-up, reported a mean reduction of 32 mmHg in SBP and 12 mmHg in DBP. In absolute terms, the reduction was greater than in our study, but comparison between the results of the two studies is difficult for various reasons: our sample was smaller (approximately half); baseline BP levels were higher in Symplicity HTN-212 (SBP 178 vs. 174 mmHg and DBP 97 vs. 89 mmHg), making a greater fall in BP more likely; and particularly importantly, drug therapy was not maintained throughout follow-up in our study population, which may have affected our results. The above may also explain the differences found in BP values on 24-hour ABPM: in contrast to the Symplicity HTN-2 study,12 in which 24-hour ABPM values fell significantly six months after RDN, our study found a statistically significant reduction in diastolic load only, even though mean SBP and DBP decreased (by 5 and 3 mmHg, respectively). Besides the short-term benefits demonstrated in the present study, the long-term efficacy of RDN has been reported in up to 36 months of follow-up.14
With regard to echocardiographic parameters, there was a significant reduction in LVMI, in line with the results published by other groups.28 This finding is particularly important since LV hypertrophy is a marker of subclinical target organ damage and is associated with cardiovascular events.29 Furthermore, regression of LV hypertrophy as a result of better HTN control has been shown to improve prognosis.30 However, unlike in previous studies,28 our analysis found no significant improvement in systolic or diastolic function after RDN, probably due to the small sample size.
Some questions remain concerning the applicability of RDN. Careful patient selection, thorough investigation of the reasons behind nonadherence to drug therapy and exclusion of white coat HTN are essential aspects that require improvement. A recent study in 84 hypertensive patients assessing adherence to therapy through measurement of serum antihypertensive drug levels showed that 34.5% had no detectable drugs in the circulation and that 65.5% met criteria for nonadherence.31 Against this background, it is difficult to determine whether the impact of RDN on BP levels is due to the intervention itself, possible improved compliance with therapy, or even a placebo effect, as seen in various areas of medicine. The Symplicity HTN-3 study,32 currently in progress, one endpoint of which is ABPM assessment, will help to answer some of these questions. On the other hand, sympathetic activity may vary from patient to patient, and it is therefore crucial to identify objective parameters that will predict the response to RDN, enabling those with greater potential to respond to be selected and possible non-responders to be identified. The number of applications and the radiofrequency dose may in the future be established on an individual basis, adapted to the specific characteristics of each patient. Another question concerns sympathetic nervous system activation in the different stages of HTN. It is possible that sympathetic modulation in the early stages of HTN has a greater beneficial effect and can influence the natural history of the disease. Finally, further studies are required to determine the impact of RDN on morbidity and mortality in patients with resistant HTN, as well as to validate the cost-effectiveness of the technique, although preliminary data suggest that this is favorable.33 The expectations surrounding RDN are reflected by the fact that several endovascular intervention systems are currently under development, clinical trials and registries of which will increase knowledge in this area and answer some of the above questions, leading to improvements in patient comfort and the procedure's safety and efficacy, which are essential for more widespread adoption of the technique.34
LimitationsThe present study has certain limitations. Firstly, the study population was small, which means it is not possible to draw definitive conclusions as to the efficacy and safety of RDN, make comparisons between the different RDN systems used in terms of safety, or determine the demographic and clinical profile of patients who will not respond to RDN. The fact that control renal artery angiography was not systematically performed during follow-up prevented a full assessment of the medium- to long-term safety of radiofrequency ablation. It was also not possible to compare the efficacy of the various devices used, since all the patients with complete six-month follow-up were treated with the Symplicity® system. Lastly, changes were made in drug therapy during follow-up, which may have influenced assessment of the efficacy of RDN, leading to underestimation of its effect on the various parameters studied.
ConclusionIn this cohort of patients with resistant essential HTN, RDN was safe and effective at six-month follow-up, with significant reductions in office SBP and DBP and a significant decrease in the number of antihypertensive drugs prescribed. In addition, RDN significantly reduced LVMI, a known marker of target organ damage. RDN thus appears to be a valid option for patients with resistant HTN, with benefits beyond improved BP control. Nevertheless, randomized studies with larger populations are required to assess the impact of this intervention on clinical events in the long term.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Dores H, de Sousa Almeida M, de Araújo Gonçalves P, Branco P, Gaspar A, Sousa H, et al. Desnervação renal em doentes com hipertensão arterial resistente: resultados aos seis meses de seguimento. Rev Port Cardiol. 2014;33:197–204.