We present a case of acute heart failure as the first manifestation of Graves’ disease. It illustrates some of its cardiovascular complications, particularly atrial fibrillation, pulmonary hypertension and heart failure. This case report highlights the importance of considering hyperthyroidism as a cause of idiopathic pulmonary hypertension, and demonstrates the potential reversibility of its complications.
Apresenta-se um caso de insuficiência cardíaca aguda como manifestação inicial de doença de Graves, ilustrando-se as suas complicações cardiovasculares: fibrilhação auricular, hipertensão pulmonar e insuficiência cardíaca. O caso mostra como as complicações do hipertiroidismo são potencialmente reversíveis, analisando-se assim a importância da sua inclusão nas causas de hipertensão pulmonar não explicada.
We present a case of acute heart failure (HF) in a 41-year-old woman who went to the emergency department due to progressive dyspnea. At admission, she was in New York Heart Association (NYHA) class IV, with lower limb edema of around one week's duration. She reported no fever, cough, hemoptysis, chest pain, weight loss or joint pain. The patient, a farmer, had been previously healthy and took no regular medication, did not smoke or drink alcohol, and had not traveled in the recent past.
Her pulse was irregular, heart rate 115 bpm, blood pressure 147/86 mmHg; she was apyretic and peripheral oxygen saturation was 97% in room air. Physical examination revealed clammy skin and fine hair, exophthalmos and diffuse enlargement of the thyroid gland with thrill but no palpable nodules (Figure 1).
Jugular venous distension of 8cm at 45° was visible. Cardiac auscultation showed no murmurs, and pulmonary auscultation revealed rales in both lung bases. The patient had painless hepatomegaly 2cm below the ribcage and bilateral lower limb edema up to the knee.
Initial clinical investigation included laboratory tests, which revealed anemia, negative D-dimers and myocardial necrosis markers, and elevated pro-BNP (Table 1), and the chest X-ray showed increased cardiothoracic index (Figure 2).
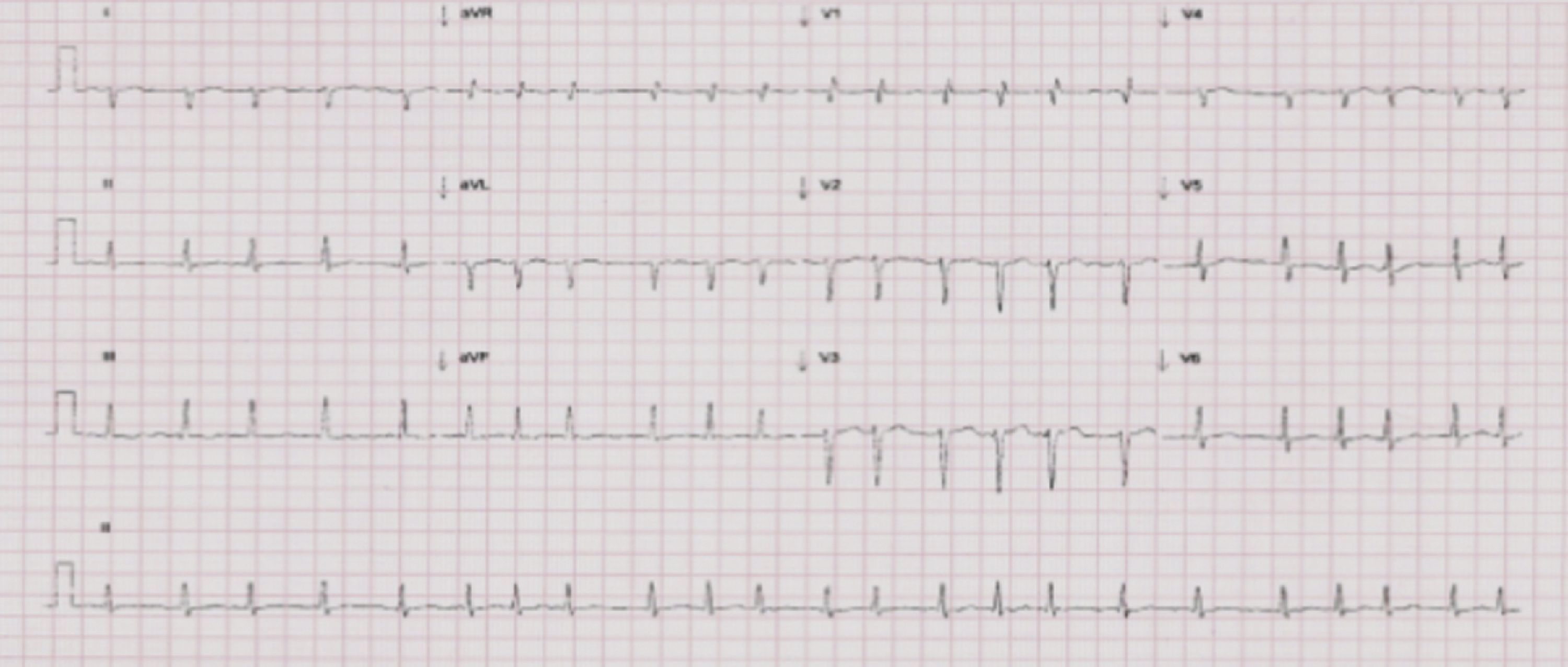
The setting was interpreted as acute HF, and etiological investigation included electrocardiography (ECG) and transthoracic echocardiography (TTE). The ECG showed atrial fibrillation (AF) and a mean ventricular rate of 130 bpm, but no ST-T segment abnormalities (Figure 3).
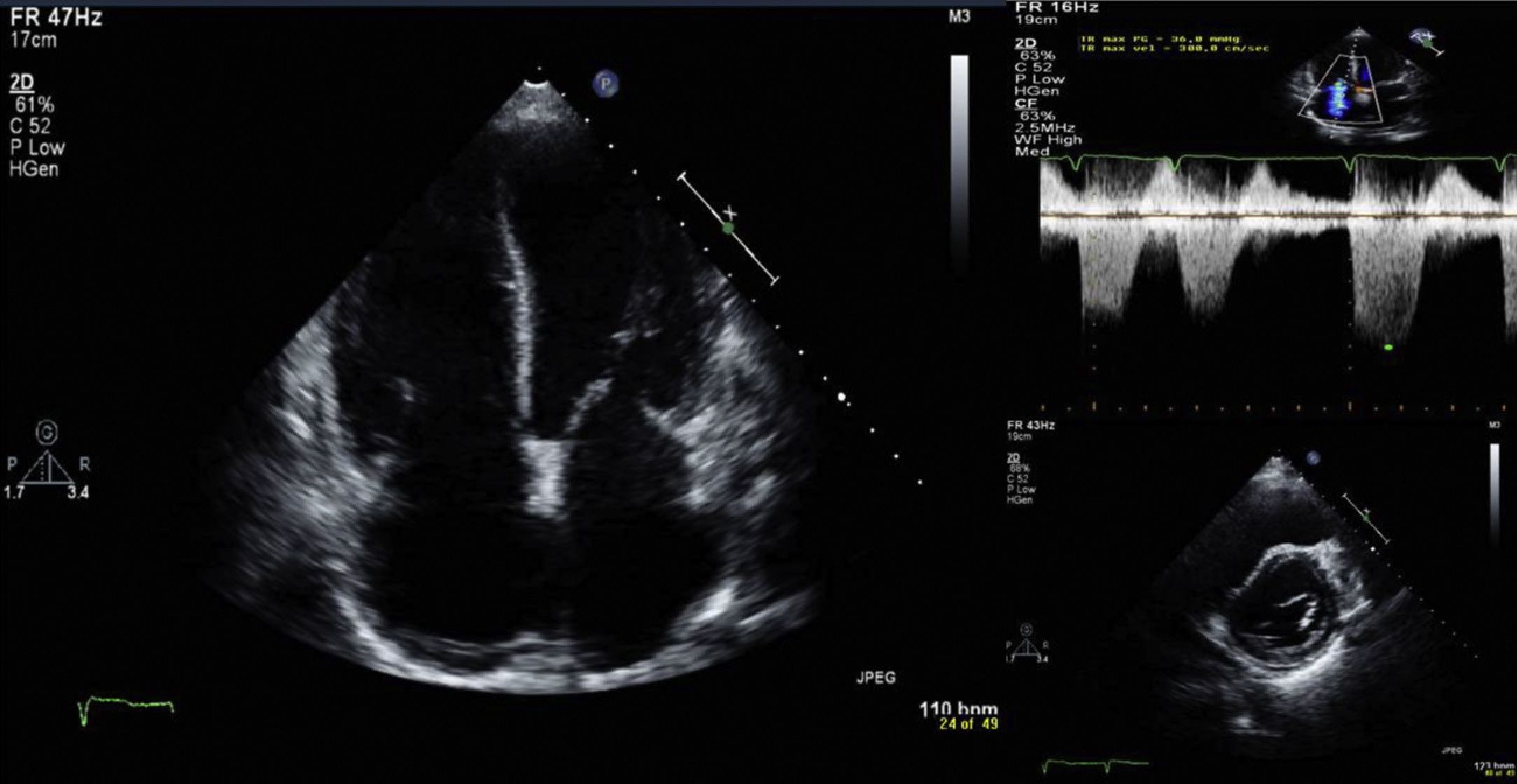
TTE, performed with the patient in AF, showed degenerative changes in valve structures with no significant hemodynamic compromise and mild to moderate tricuspid regurgitation, with pulmonary artery systolic pressure (PASP) estimated at 36 mmHg and right atrial pressure at 20 mmHg. There was mild right chamber dilatation (right atrial area 26cm2), with flattening of the interventricular septum secondary to right ventricular (RV) overload (Figure 4), RV systolic function at the lower normal limit (tricuspid annular plane systolic excursion 1.7cm; S wave 13cm/s) and mild inferior vena caval dilatation (28mm) with reduced respiratory variation, all compatible with pulmonary hypertension (PH). There was also moderate left atrial dilatation (area 31cm2), with normal left ventricular (LV) dimensions (end-diastolic diameter 52mm) and ventricular wall thickness. LV systolic function (LVSF) was mildly impaired, with a mean ejection fraction of 46% and increased LV filling pressures (septal E/E′ 21.9).
Findings on physical examination suggested thyroid disease, and thyroid function tests were compatible with hyperthyroidism: free T4 100pmol/ml (normal 12–22) and thyroid-stimulating hormone (TSH) <0.005μU/ml (0.27–4.20). TSH-receptor antibodies (TRAb) were elevated (12.28U/l; normal <1) and thyroid ultrasound showed diffuse enlargement of the thyroid gland, which was generally heterogeneous in texture with solid hyperechogenic nodules.
The combination of diffuse thyroid enlargement, laboratory evidence of thyrotoxicosis, exophthalmos and positive TRAb led to a diagnosis of Graves’ disease (GD), complicated by AF, PH and HF. Treatment was begun with metibasol (30mg/day), propranolol (60mg/day), enalapril (5mg/day), furosemide (60mg/day) and aspirin (150mg/day). It was decided to institute antiplatelet therapy since older age is the main risk factor for embolic phenomena in hyperthyroidism. In younger patients, the risks of anticoagulation are greater than its potential benefits.1
After six months of medical therapy, and normal thyroid function for three months (TSH 2.13μU/ml and free T4 0.881pmol/l), the patient showed improvement in dyspnea (from NYHA class IV to II) and no peripheral edema. The ECG showed sinus rhythm, with heart rate of 49 bpm. Reassessment by TTE showed PASP of 38 mmHg, RV dimensions at the upper normal limit, and mean ejection fraction of 54%. Despite normal thyroid function, the patient still had an enlarged thyroid gland and positive TRAb, predictive of recurrence.2 She has poor tolerance of antithyroid drugs and is reluctant to undergo iodine therapy, and has been referred for surgical consultation with a view to thyroidectomy.
DiscussionThe role of hyperthyroidism as a possible factor in decompensated heart disease, cardiomegaly, AF and HF is well established.3 In cardiovascular terms, the most common changes arising from hyperthyroidism are increased blood volume, decreased peripheral vascular resistance, and increased resting heart rate and LV contractility, which together result in a hyperdynamic circulatory state. The manifestations usually include palpitations, tachycardia and exercise intolerance. Although sinus tachycardia is the most common rhythm disturbance, AF is found in 10–25% of patients.1 In the case presented, AF with rapid ventricular rate (130 bpm at admission) in the context of hyperthyroidism may explain the transient LV dysfunction observed (tachycardiomyopathy). At six-month assessment following treatment, the patient was in sinus rhythm, with a heart rate of 49 bpm and preserved LVSF.
GD accounts for 60–80% of cases of hyperthyroidism, with a female/male ratio of 5–10:1 and peak incidence between the ages of 40 and 60,4 as in the case presented. PH is associated with various autoimmune diseases, including GD, but the distinguishing feature of this case was the association of GD and PH with symptomatic right HF, which is uncommon, particularly as the first manifestation of GD.3,5,6 PH is currently defined as mean PASP of >25 mmHg at rest as assessed by right heart catheterization. However, there is a strong correlation between PASP estimated by this technique and by TTE.7 TTE is thus considered a useful noninvasive modality for initial assessment of PH, and is the first-line exam in the diagnostic algorithm for PH in the 2011 European Society of Cardiology (ESC) guidelines.8 Its main advantage is that it can not only estimate PASP based on the peak velocity of tricuspid regurgitant flow, but also assess indirect signs of PH such as right chamber dilatation and abnormal interventricular septal motion, which increases the likelihood of a correct diagnosis, as well as being particularly useful in detecting the causes of PH. In the case presented, TTE showed impaired LVSF as a possible contributing factor to PH (group 2 in the ESC guidelines: PH due to LV dysfunction). However, there appeared to be some discrepancy between the degree of LV dysfunction, clinical severity and estimated degree of PH. Thus, in accordance with the ESC diagnostic algorithm, further investigation of the most common causes of PH was warranted. Although the algorithm does not include catheterization at this stage, it could have provided accurate assessment of the mechanism involved by determining pulmonary artery wedge pressure.
The findings from physical examination pointed towards hyperthyroidism and laboratory tests confirmed a diagnosis of GD. Various mechanisms lie behind the pathogenesis of PH in GD; unlike their effects at the peripheral level, thyroid hormones do not reduce pulmonary vascular resistance, and there is endothelial damage caused by the autoimmune process.9 We therefore interpret the clinical setting as PH secondary to GD, exacerbated by LV dysfunction caused by tachycardiomyopathy in the context of AF with rapid ventricular rate due to hyperthyroidism. Other common causes appear less likely, since there was no known history of pulmonary disease, the chest X-ray showed no relevant alterations, and there was no hypoxia, excluding ESC group 3 (PH due to pulmonary disease and/or hypoxia); negative D-dimers and low pre-test probability associated with resolution of the setting following antithyroid therapy make group 4 (PH due to thromboembolism) unlikely.
One aspect worth highlighting in this case is the reversibility of PH associated with GD after restoration of normal thyroid function. In a recent study involving 64 patients, the prevalence of PH was 28%, with full recovery after antithyroid treatment in all but one patient.10 In the case presented, after three months of normal thyroid function, reassessment showed signs of reversibility of PH and symptomatic right HF, with marked clinical improvement and favorable echocardiographic evolution.
ConclusionsThis case shows the importance of considering hyperthyroidism as a cause of idiopathic HF and PH, since it is treatable and its complications may be reversible.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Baptista A, et al. Hipertensão pulmonar, insuficiência cardíaca e hipertiroidismo: caso clínico. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.12.005.