There is still debate concerning the impact of left ventricular end-diastolic pressure (LVEDP) on long-term prognosis after an acute coronary syndrome (ACS).
ObjectiveTo assess LVEDP and its prognostic implications in ACS patients with left ventricular ejection fraction (LVEF) ≥40%.
MethodsWe performed a prospective, longitudinal study of 1329 ACS patients from a single center between 2004 and 2006. LVEDP was assessed at the beginning of the coronary angiogram. Patients with LVEF >40% were included (n=489). The population was divided into three groups: A — LVEDP ≤19mmHg (n=186); B — LVEDP >19 and ≤27mmHg (n=172); and C — LVEDP >27mmHg (n=131). The primary endpoint of the analysis was readmission for congestive heart failure in the year following the index admission.
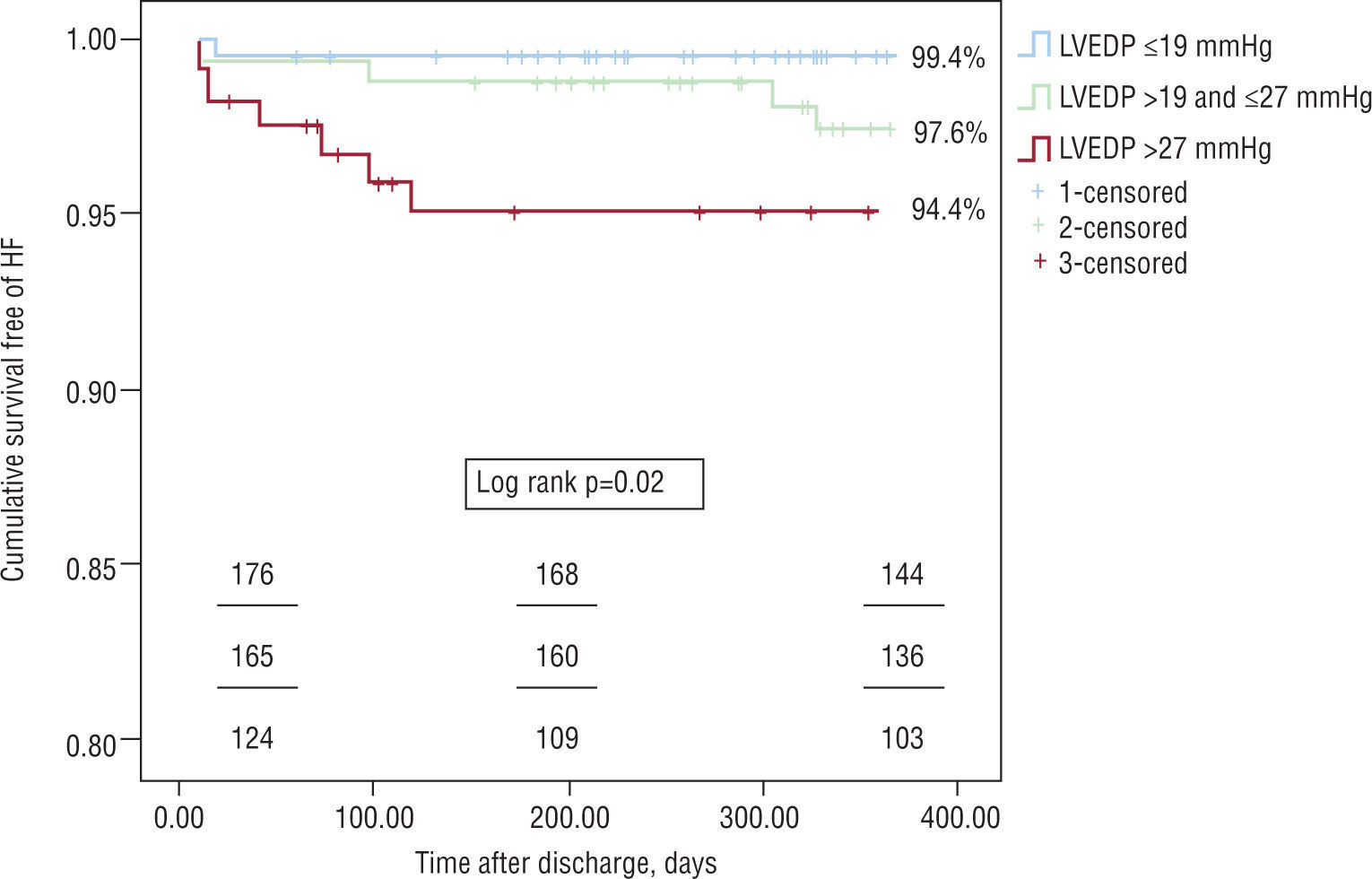
ResultsMean LVEDP was 22.8±7.8mmHg. The groups were similar age, gender, cardiovascular risk factors, cardiovascular history, and medication prior to admission. There was an association between higher LVEDP and: admission for ST-elevation acute myocardial infarction (35.4 vs. 45.9 vs. 56.7%, p<0.01), higher peak levels of cardiac biomarkers, and lower LVEF (56.5±7.0 vs. 55.3±7.6 vs. 53.0±7.5%, p<0.01). There were no significant differences between the groups in terms of coronary anatomy, medical therapy during hospital stay and at discharge, or in-hospital mortality. With regard to the primary endpoint, cumulative freedom from congestive heart failure was higher in group A patients (99.4 vs. 97.6 vs. 94.4%, log rank p=0.02). In a multivariate Cox regression model, a 5-mmHg increase in LVEDP (HR 1.97, 95% CI 1.10–3.54, p=0.02) remained an independent predictor of the primary endpoint when adjusted for age, systolic function, atrial fibrillation, peak troponin I, renal function, and prescription of diuretics and beta-blockers.
ConclusionIn selected population LVEDP was a significant prognostic marker of future admission for congestive heart failure.
Permanecem dúvidas sobre o impacto prognóstico a longo prazo da pressão telediastólica do ventrículo esquerdo (PTDVE) no contexto de uma síndrome coronária aguda (SCA).
ObjectivoCaracterizar a PTDVE e o seu impacto prognóstico numa população de doentes com SCA e fracção de ejecção ventricular esquerda (FEVE)≥a 40%.
População e métodosEstudo prospectivo, longitudinal e contínuo de 1329 doentes admitidos (n=489) numa unidade de cuidados intensivos coronários entre 2004 e 2006. Foram seleccionados os doentes submetidos a uma estratégia invasiva, no qual foi determinada a PTDVE, com FEVE≥40%. A população foi divida em três grupos: A — PTDVE≤19mmHg (n=186); grupo B — PTDVE > 19≤27mmHg (n=172) e ; grupo C — PTDVE > 27mmHg (n=131). O resultado primário desta análise foi a readmissão por insuficiência cardíaca congestiva (ICC) no ano seguinte à SCA.
ResultadosA PTDVE média da população foi de 22,8mmHg±7,8mmHg. Os grupos eram homogéneos entre si no que disse respeito ao género, idade, factores de risco cardiovascular, história cardiovascular e medicação prévia à admissão. Quanto maior a PTDVE maior a probabilidade de uma admissão por enfarte agudo do miocárdio com supradesnivelamento do segmento ST (35,4 versus 45,9 versus 56,7 %, p<0,01), maior a libertação de marcadores de necrose miocárdica, e menor a FEVE (56,5±7,0 versus 55,3±7,6 versus 53,0±7,5 %, p<0,01). Não foram detectadas diferenças entre os grupos relativamente à anatomia coronária, revascularização, terapêutica médica intra-hospitalar e à data de alta, e mortalidade intra-hospitalar. Quanto ao resultado primário desta análise, a sobrevida livre de readmissão por ICC foi superior para os doentes com menor PTDVE — 99,4 versus 97,6 versus 94,4%, log rank p=0,02. A PTDVE (sob a forma de um incremento de 5 em 5mmHg), foi um preditor independente para a readmissão por ICC, quando ajustada para as seguintes variáveis: idade (incremento de 10 em 10 anos), FEVE (incremento de 5 em 5%), pico de troponina I (incremento de 5 em 5 U/L) insuficiência renal (taxa de filtração glomerular menor a 60ml/min), fibrilhação auricular, prescrição de diuréticos às 24 horas, e de beta-bloqueante à data de alta. Por cada 5mmHg de aumento da PTDVE o risco de uma readmissão por ICC um ano após a SCA aumentou 1,97 vezes (RR 1,97, IC 95% 1,10–3,54, p=0,02).
ConclusãoNa população referida a PTDVE teve um impacto prognóstico importante a longo prazo relativamente à readmissão, hospitalar por ICC.
Left ventricular end-diastolic pressure (LVEDP) is directly related to left ventricular (LV) compliance and intravascular volume and pressure1, and one of the main consequences of LV diastolic dysfunction is increased LV filling pressures2.
In the context of coronary artery disease, elevated filling pressures can be caused by acute ischemia, an infarction scar, or myocardial hibernation. Acute ischemia results in an upward and rightward shift of the diastolic pressure-volume curve, which may be due to various factors: calcium overload in cardiomyocytes resulting in late activation and incomplete relaxation, pericardial constriction due to increased atrial and ventricular volume, intraand interventricular asynchrony and papillary muscle dysfunction3.
Elevated filling pressures are known to have a short-term prognostic impact in patients with acute coronary syndromes (ACS), as demonstrated by Forrester4 at the end of the 1970s and reconfirmed many years later5. The importance of LVEDP in the development of heart failure (HF) within 30 days of ACS has also been shown in patients with ST-elevation myocardial infarction6. On the other hand, a substudy of the Survival and Ventricular Enlargement (SAVE) trial analyzed the importance of filling pressures in late development of HF in a population of patients with left ventricular ejection fraction (LVEF) of less than 40% and a history of ACS, and concluded that LVEDP was not an independent predictor of subsequent HF7.
In view of the uncertainty surrounding the importance of LVEDP in the long term, the present study aimed to assess this parameter during the acute phase of ACS in consecutive patients from a single center treated by an invasive strategy and with no significant left ventricular systolic dysfunction, in order to determine whether LVEDP has prognostic impact on in-hospital outcomes and during one-year follow-up.
MethodsWe performed a prospective, longitudinal and observational study (prospective data collection with retrospective outcome analysis) of 1459 consecutive admissions for ACS between May 10, 2004 and December 31, 2006 to a coronary care unit of a single center. Of these, 1329 patients were identified, 130 readmissions being excluded. Of this total, 786 treated by an invasive strategy during the index admission were selected, for 675 of whom LVEDP data were available. Patients with LVEF of less than 40% were excluded, leaving a total of 489.
LVEDP was assessed in the hemodynamic laboratory immediately prior to left ventriculography, measured 50 ms after the beginning of the QRS complex, usually coinciding with the R wave. The decision to perform ventriculography and LVEDP assessment was the responsibility of the hemodynamicist on duty.
Within 24 hours of the invasive study, LV systolic function was assessed by echocardiography, LVEF being calculated by the Simpson method. All patients with LVEF of less than 40% were excluded from the analysis.
In accordance with the universal definition of myocardial infarction, diagnosis was made on the basis of markers of myocardial necrosis (troponin I) above the 99th percentile, together with evidence of myocardial ischemia8. ST-elevation myocardial infarction (STEMI) was defined as anginal chest pain, together with de novo ST elevation of >1 mm in two contiguous leads. Non-ST elevation myocardial infarction (NSTEMI) was defined as anginal chest pain lasting more than five minutes, with or without electrocardiographic (ECG) alterations (ST-segment depression or T-wave inversion). A diagnosis of unstable angina was based on the presence of new-onset anginal chest pain (at least CCS class III), worsening angina or rest angina, with or without associated ECG alterations.
For patients with NSTEMI or unstable angina, the decision on whether to adopt an invasive or a conservative strategy was based on clinical data and the results of ischemia testing, as previously described9.
Standard records from the hospital stay were analyzed, including demographic, clinical, electrocardiographic, echocardiographic and laboratory data, therapy prescribed during hospital stay, occurrence and type of in-hospital complications, treatment in the hemodynamic laboratory, duration of hospital stay and medication at discharge.
Patients were followed for a year after discharge, by telephone or by consulting hospital records. The primary endpoint in the present analysis was readmission for congestive heart failure (CHF). The secondary endpoint was the combined outcome of major adverse cardiac events (MACE) (death from cardiovascular cause, nonfatal infarction, readmission for unstable angina, or unscheduled percutaneous coronary intervention) and all-cause mortality in the first year.
The population was divided into three groups based on the distribution of percentiles (P33) of LVEDP values: group A – LVEDP ≤19 mmHg (n=186); group B – LVEDP >19 and ≤27 mmHg (n=172); and group C – LVEDP >27 mmHg (n=131).
Statistical analysisContinuous variables were presented as means ± standard deviation, and analysis of variance was performed using the one-way ANOVA test. Categorical variables were expressed as absolute frequencies and percentages, and the chi-square test was used to compare groups. The chi-square test for linear trend was also used to clarify the type of associations found. A value of p<0.05 was considered statistically significant.
Kaplan-Meier survival curves were constructed to assess survival free of readmission for CHF and the groups were compared using the log rank test. The follow-up period analyzed began at hospital discharge and ended 12 months later.
A multivariate Cox regression model was constructed for the study's primary endpoint, based on a level of significance of 0.05 in univariate analysis.
The statistical analysis was performed using SPSS version 15 (SPSS Inc., Chicago, IL).
ResultsLVEDP data were available for 489 patients. Mean LVEDP was 22.8±7.8 mmHg, and around 80% presented LVEDP of >17 mmHg. Mean age was 62.4±12.4 years, and 359 patients were male.
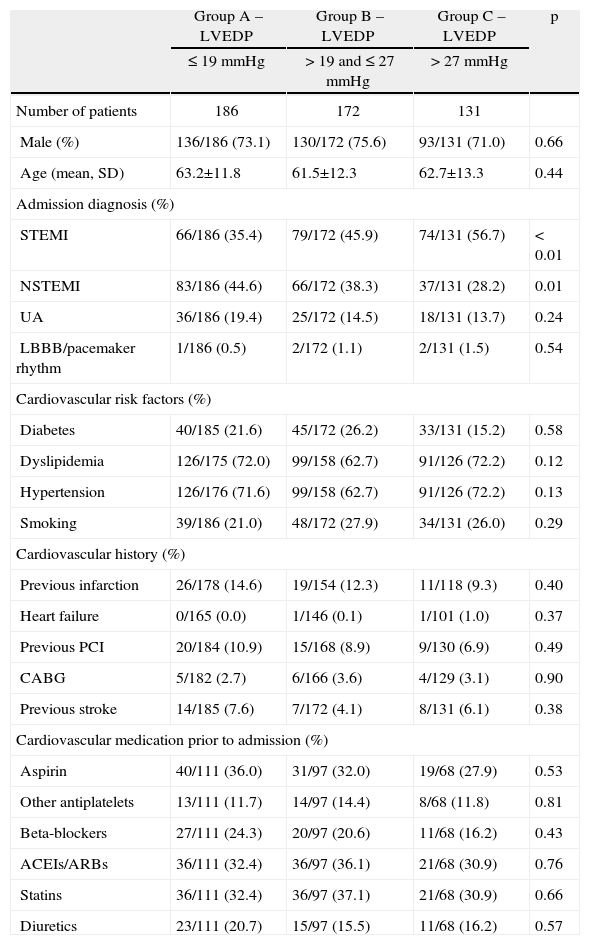
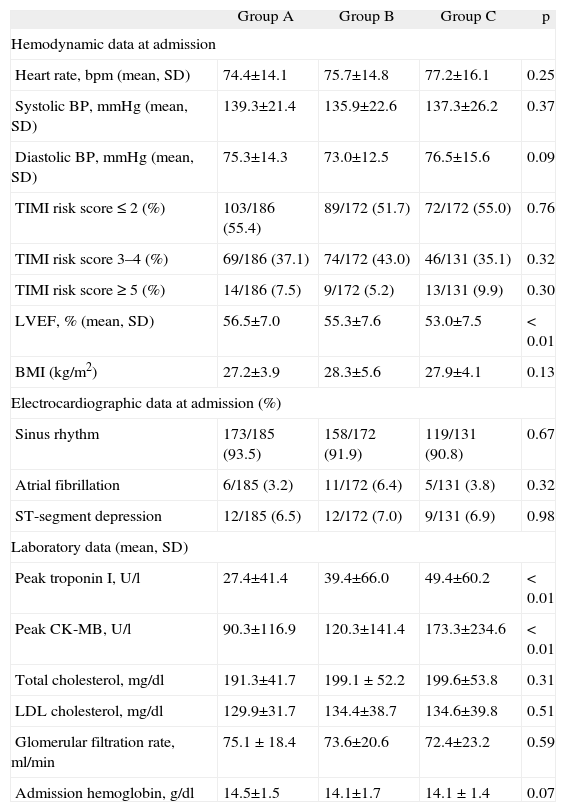
The demographic, clinical, laboratory and electrocardiographic characteristics of the three groups are shown in Tables 1 and 2.
General characteristics of the study population
| Group A – LVEDP | Group B – LVEDP | Group C – LVEDP | p | |
| ≤19 mmHg | >19 and ≤27 mmHg | >27 mmHg | ||
| Number of patients | 186 | 172 | 131 | |
| Male (%) | 136/186 (73.1) | 130/172 (75.6) | 93/131 (71.0) | 0.66 |
| Age (mean, SD) | 63.2±11.8 | 61.5±12.3 | 62.7±13.3 | 0.44 |
| Admission diagnosis (%) | ||||
| STEMI | 66/186 (35.4) | 79/172 (45.9) | 74/131 (56.7) | <0.01 |
| NSTEMI | 83/186 (44.6) | 66/172 (38.3) | 37/131 (28.2) | 0.01 |
| UA | 36/186 (19.4) | 25/172 (14.5) | 18/131 (13.7) | 0.24 |
| LBBB/pacemaker rhythm | 1/186 (0.5) | 2/172 (1.1) | 2/131 (1.5) | 0.54 |
| Cardiovascular risk factors (%) | ||||
| Diabetes | 40/185 (21.6) | 45/172 (26.2) | 33/131 (15.2) | 0.58 |
| Dyslipidemia | 126/175 (72.0) | 99/158 (62.7) | 91/126 (72.2) | 0.12 |
| Hypertension | 126/176 (71.6) | 99/158 (62.7) | 91/126 (72.2) | 0.13 |
| Smoking | 39/186 (21.0) | 48/172 (27.9) | 34/131 (26.0) | 0.29 |
| Cardiovascular history (%) | ||||
| Previous infarction | 26/178 (14.6) | 19/154 (12.3) | 11/118 (9.3) | 0.40 |
| Heart failure | 0/165 (0.0) | 1/146 (0.1) | 1/101 (1.0) | 0.37 |
| Previous PCI | 20/184 (10.9) | 15/168 (8.9) | 9/130 (6.9) | 0.49 |
| CABG | 5/182 (2.7) | 6/166 (3.6) | 4/129 (3.1) | 0.90 |
| Previous stroke | 14/185 (7.6) | 7/172 (4.1) | 8/131 (6.1) | 0.38 |
| Cardiovascular medication prior to admission (%) | ||||
| Aspirin | 40/111 (36.0) | 31/97 (32.0) | 19/68 (27.9) | 0.53 |
| Other antiplatelets | 13/111 (11.7) | 14/97 (14.4) | 8/68 (11.8) | 0.81 |
| Beta-blockers | 27/111 (24.3) | 20/97 (20.6) | 11/68 (16.2) | 0.43 |
| ACEIs/ARBs | 36/111 (32.4) | 36/97 (36.1) | 21/68 (30.9) | 0.76 |
| Statins | 36/111 (32.4) | 36/97 (37.1) | 21/68 (30.9) | 0.66 |
| Diuretics | 23/111 (20.7) | 15/97 (15.5) | 11/68 (16.2) | 0.57 |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; CABG: coronary artery bypass grafting; LBBB: left bundle branch block; LVEDP: left ventricular end-diastolic pressure; NSTEMI: non-ST elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; UA: unstable angina.
Hemodynamic, electrocardiographic and laboratory data
| Group A | Group B | Group C | p | |
| Hemodynamic data at admission | ||||
| Heart rate, bpm (mean, SD) | 74.4±14.1 | 75.7±14.8 | 77.2±16.1 | 0.25 |
| Systolic BP, mmHg (mean, SD) | 139.3±21.4 | 135.9±22.6 | 137.3±26.2 | 0.37 |
| Diastolic BP, mmHg (mean, SD) | 75.3±14.3 | 73.0±12.5 | 76.5±15.6 | 0.09 |
| TIMI risk score ≤2 (%) | 103/186 (55.4) | 89/172 (51.7) | 72/172 (55.0) | 0.76 |
| TIMI risk score 3–4 (%) | 69/186 (37.1) | 74/172 (43.0) | 46/131 (35.1) | 0.32 |
| TIMI risk score ≥5 (%) | 14/186 (7.5) | 9/172 (5.2) | 13/131 (9.9) | 0.30 |
| LVEF, % (mean, SD) | 56.5±7.0 | 55.3±7.6 | 53.0±7.5 | <0.01 |
| BMI (kg/m2) | 27.2±3.9 | 28.3±5.6 | 27.9±4.1 | 0.13 |
| Electrocardiographic data at admission (%) | ||||
| Sinus rhythm | 173/185 (93.5) | 158/172 (91.9) | 119/131 (90.8) | 0.67 |
| Atrial fibrillation | 6/185 (3.2) | 11/172 (6.4) | 5/131 (3.8) | 0.32 |
| ST-segment depression | 12/185 (6.5) | 12/172 (7.0) | 9/131 (6.9) | 0.98 |
| Laboratory data (mean, SD) | ||||
| Peak troponin I, U/l | 27.4±41.4 | 39.4±66.0 | 49.4±60.2 | <0.01 |
| Peak CK-MB, U/l | 90.3±116.9 | 120.3±141.4 | 173.3±234.6 | <0.01 |
| Total cholesterol, mg/dl | 191.3±41.7 | 199.1 ±52.2 | 199.6±53.8 | 0.31 |
| LDL cholesterol, mg/dl | 129.9±31.7 | 134.4±38.7 | 134.6±39.8 | 0.51 |
| Glomerular filtration rate, ml/min | 75.1 ±18.4 | 73.6±20.6 | 72.4±23.2 | 0.59 |
| Admission hemoglobin, g/dl | 14.5±1.5 | 14.1±1.7 | 14.1 ±1.4 | 0.07 |
BMI: body mass index; BP: blood pressure; LVEF: left ventricular ejection fraction.
The groups were similar in age, gender, cardiovascular risk factors, cardiovascular history and medication prior to admission. With regard to admission diagnosis, there was a progressively stronger association between higher LVEDP and STEMI in the three groups (35.4 vs. 45.9 vs. 56.7%, p<0.01), the opposite being found with NSTEMI (44.6 vs. 38.3 vs. 28.2%, p=0.01) (Table 1).
There was a weak but significant negative correlation between LVEF and LVEDP (r=−0.22, p<0.001), although there was a significant progressive decrease in LVEF in the three groups (56.5±7.0 vs. 55.3±7.6 vs. 53.0±7.5%, p<0.01). There was also an increasing association with markers of myocardial necrosis, particularly peak troponin I (27.4±41.4 vs. 39.4±66.0 vs. 49.4±60.2 U/l, p<0.01) and CK-MB (Table 2).
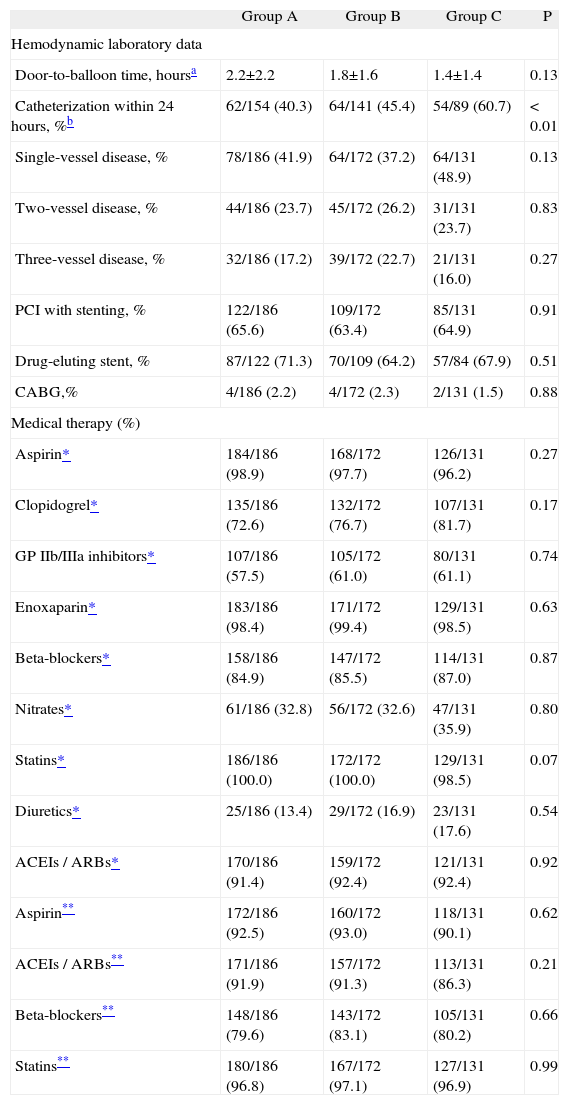
With regard to hemodynamic laboratory data, an association was found between time to catheterization and LVEDP, especially in the group with non-ST elevation ACS. There was no statistically significant correlation between coronary disease severity, percutaneous intervention or surgical revascularization and LVEDP in the three groups (Table 3).
Hemodynamic laboratory data and medical therapy during hospital stay and at discharge
| Group A | Group B | Group C | P | |
| Hemodynamic laboratory data | ||||
| Door-to-balloon time, hoursa | 2.2±2.2 | 1.8±1.6 | 1.4±1.4 | 0.13 |
| Catheterization within 24 hours, %b | 62/154 (40.3) | 64/141 (45.4) | 54/89 (60.7) | <0.01 |
| Single-vessel disease, % | 78/186 (41.9) | 64/172 (37.2) | 64/131 (48.9) | 0.13 |
| Two-vessel disease, % | 44/186 (23.7) | 45/172 (26.2) | 31/131 (23.7) | 0.83 |
| Three-vessel disease, % | 32/186 (17.2) | 39/172 (22.7) | 21/131 (16.0) | 0.27 |
| PCI with stenting, % | 122/186 (65.6) | 109/172 (63.4) | 85/131 (64.9) | 0.91 |
| Drug-eluting stent, % | 87/122 (71.3) | 70/109 (64.2) | 57/84 (67.9) | 0.51 |
| CABG,% | 4/186 (2.2) | 4/172 (2.3) | 2/131 (1.5) | 0.88 |
| Medical therapy (%) | ||||
| Aspirin* | 184/186 (98.9) | 168/172 (97.7) | 126/131 (96.2) | 0.27 |
| Clopidogrel* | 135/186 (72.6) | 132/172 (76.7) | 107/131 (81.7) | 0.17 |
| GP IIb/IIIa inhibitors* | 107/186 (57.5) | 105/172 (61.0) | 80/131 (61.1) | 0.74 |
| Enoxaparin* | 183/186 (98.4) | 171/172 (99.4) | 129/131 (98.5) | 0.63 |
| Beta-blockers* | 158/186 (84.9) | 147/172 (85.5) | 114/131 (87.0) | 0.87 |
| Nitrates* | 61/186 (32.8) | 56/172 (32.6) | 47/131 (35.9) | 0.80 |
| Statins* | 186/186 (100.0) | 172/172 (100.0) | 129/131 (98.5) | 0.07 |
| Diuretics* | 25/186 (13.4) | 29/172 (16.9) | 23/131 (17.6) | 0.54 |
| ACEIs / ARBs* | 170/186 (91.4) | 159/172 (92.4) | 121/131 (92.4) | 0.92 |
| Aspirin** | 172/186 (92.5) | 160/172 (93.0) | 118/131 (90.1) | 0.62 |
| ACEIs / ARBs** | 171/186 (91.9) | 157/172 (91.3) | 113/131 (86.3) | 0.21 |
| Beta-blockers** | 148/186 (79.6) | 143/172 (83.1) | 105/131 (80.2) | 0.66 |
| Statins** | 180/186 (96.8) | 167/172 (97.1) | 127/131 (96.9) | 0.99 |
ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; CABG: coronary artery bypass grafting; GP: glycoprotein; PCI: percutaneous coronary intervention.
No statistically significant differences were observed between the groups in terms of pharmacological therapy within 24 hours of admission or at discharge (Table 3).
In-hospital outcome and follow-upThe percentage of individuals lost to follow-up was 4.7%, with data available for 465 patients.
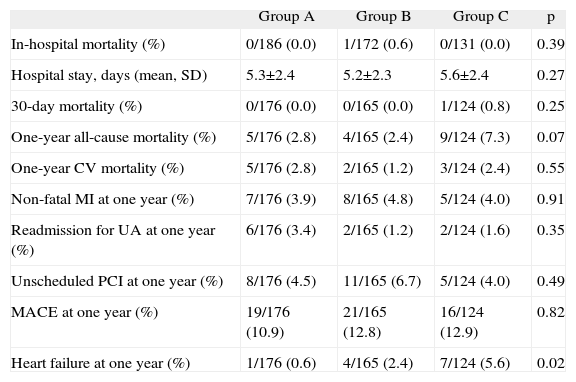
In-hospital mortality was similar in the three groups, as were 30-day and one-year mortality, and the combined outcome of MACE (Table 4).
In-hospital outcomes and at one-year follow-up
| Group A | Group B | Group C | p | |
| In-hospital mortality (%) | 0/186 (0.0) | 1/172 (0.6) | 0/131 (0.0) | 0.39 |
| Hospital stay, days (mean, SD) | 5.3±2.4 | 5.2±2.3 | 5.6±2.4 | 0.27 |
| 30-day mortality (%) | 0/176 (0.0) | 0/165 (0.0) | 1/124 (0.8) | 0.25 |
| One-year all-cause mortality (%) | 5/176 (2.8) | 4/165 (2.4) | 9/124 (7.3) | 0.07 |
| One-year CV mortality (%) | 5/176 (2.8) | 2/165 (1.2) | 3/124 (2.4) | 0.55 |
| Non-fatal MI at one year (%) | 7/176 (3.9) | 8/165 (4.8) | 5/124 (4.0) | 0.91 |
| Readmission for UA at one year (%) | 6/176 (3.4) | 2/165 (1.2) | 2/124 (1.6) | 0.35 |
| Unscheduled PCI at one year (%) | 8/176 (4.5) | 11/165 (6.7) | 5/124 (4.0) | 0.49 |
| MACE at one year (%) | 19/176 (10.9) | 21/165 (12.8) | 16/124 (12.9) | 0.82 |
| Heart failure at one year (%) | 1/176 (0.6) | 4/165 (2.4) | 7/124 (5.6) | 0.02 |
CV: cardiovascular; MACE: major adverse cardiac event; MI: myocardial infarction; PCI: percutaneous coronary intervention; UA: unstable angina.
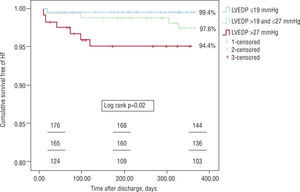
Regarding the primary endpoint of the analysis, survival free of readmission for CHF was higher in the group with lower LVEDP – 99.4 vs. 97.6 vs. 94.4%, log rank p=0.02 (Figure 1).
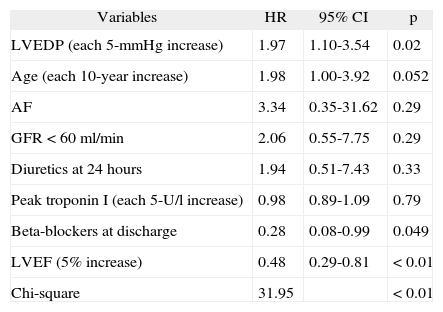
Multivariate analysisA 5-mmHg increase in LVEDP remained an independent predictor of readmission for CHF when adjusted for the following variables: age (each 10-year increase), LVEF (each 5% increase), peak troponin I (each 5 U/l increase), renal dysfunction (glomerular filtration rate <60 ml/min), atrial fibrillation, and prescription of diuretics within 24 hours of admission or beta-blockers at discharge.
Thus, in the model proposed and for the selected population, the risk of readmission for CHF one year after ACS was 1.97 times higher (RR 1.97, 95% CI 1.10-3.54, p=0.02) for each 5-mmHg increase in LVEDP.
LVEF and prescription of beta-blockers at discharge were identified as protective factors for the primary endpoint (Tables 5 and 6).
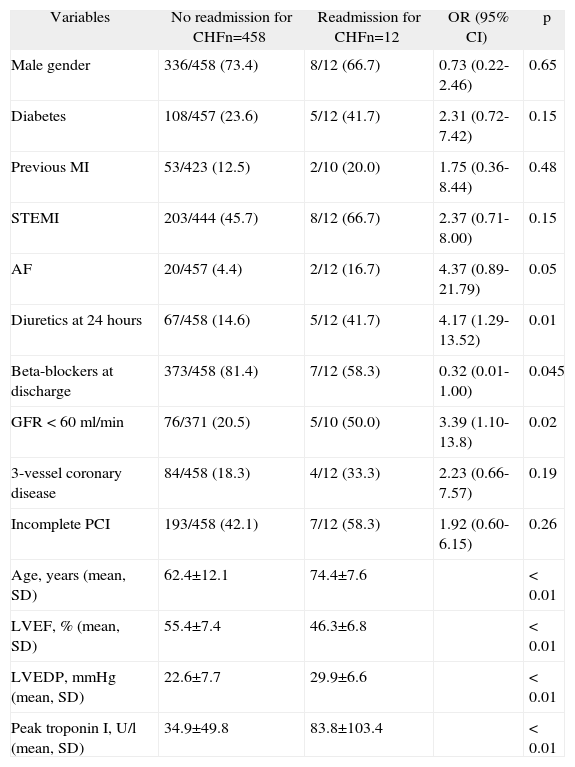
Univariate analysis: readmission for congestive heart failure within a year of ACS
| Variables | No readmission for CHFn=458 | Readmission for CHFn=12 | OR (95% CI) | p |
| Male gender | 336/458 (73.4) | 8/12 (66.7) | 0.73 (0.22-2.46) | 0.65 |
| Diabetes | 108/457 (23.6) | 5/12 (41.7) | 2.31 (0.72-7.42) | 0.15 |
| Previous MI | 53/423 (12.5) | 2/10 (20.0) | 1.75 (0.36-8.44) | 0.48 |
| STEMI | 203/444 (45.7) | 8/12 (66.7) | 2.37 (0.71-8.00) | 0.15 |
| AF | 20/457 (4.4) | 2/12 (16.7) | 4.37 (0.89-21.79) | 0.05 |
| Diuretics at 24 hours | 67/458 (14.6) | 5/12 (41.7) | 4.17 (1.29-13.52) | 0.01 |
| Beta-blockers at discharge | 373/458 (81.4) | 7/12 (58.3) | 0.32 (0.01-1.00) | 0.045 |
| GFR <60 ml/min | 76/371 (20.5) | 5/10 (50.0) | 3.39 (1.10-13.8) | 0.02 |
| 3-vessel coronary disease | 84/458 (18.3) | 4/12 (33.3) | 2.23 (0.66-7.57) | 0.19 |
| Incomplete PCI | 193/458 (42.1) | 7/12 (58.3) | 1.92 (0.60-6.15) | 0.26 |
| Age, years (mean, SD) | 62.4±12.1 | 74.4±7.6 | <0.01 | |
| LVEF, % (mean, SD) | 55.4±7.4 | 46.3±6.8 | <0.01 | |
| LVEDP, mmHg (mean, SD) | 22.6±7.7 | 29.9±6.6 | <0.01 | |
| Peak troponin I, U/l (mean, SD) | 34.9±49.8 | 83.8±103.4 | <0.01 |
AF: atrial fibrillation; CHF: congestive heart failure; CI: confidence interval; GFR: glomerular filtration rate; LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction; MI: myocardial infarction; OR: odds ratio; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
Multivariate analysis: readmission for decompensated heart failure within a year of ACS
| Variables | HR | 95% CI | p |
| LVEDP (each 5-mmHg increase) | 1.97 | 1.10-3.54 | 0.02 |
| Age (each 10-year increase) | 1.98 | 1.00-3.92 | 0.052 |
| AF | 3.34 | 0.35-31.62 | 0.29 |
| GFR <60 ml/min | 2.06 | 0.55-7.75 | 0.29 |
| Diuretics at 24 hours | 1.94 | 0.51-7.43 | 0.33 |
| Peak troponin I (each 5-U/l increase) | 0.98 | 0.89-1.09 | 0.79 |
| Beta-blockers at discharge | 0.28 | 0.08-0.99 | 0.049 |
| LVEF (5% increase) | 0.48 | 0.29-0.81 | <0.01 |
| Chi-square | 31.95 | <0.01 |
AF: atrial fibrillation; CI: confidence interval; GFR: glomerular filtration rate; HR: hazard ratio; LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction.
The present study highlights the importance of LVEDP in predicting readmission for CHF within a year of ACS in a population of patients with LVEF ≥40%. Based on the Cox model proposed, a 1.97 times higher risk for the primary endpoint was found for each increase of 5 mmHg in LVEDP.
In this cohort there was a direct correlation between LVEDP values and higher levels of myocardial necrosis markers, and, in the case of non-ST elevation ACS, time to cardiac catheterization. This finding is supported by experimental data of pacing-induced ischemia, which demonstrated decreased myocardial distensibility in this context, and hence increased filling pressures10. Interestingly, as reported by other authors, LVEDP was not directly related to the anatomical extent of coronary disease7.
In our population, LVEDP had no prognostic impact on in-hospital mortality, which may be due to the small number of patients with LVEF ≥40% treated by an invasive strategy who died in hospital. In addition, of the patients referred for catheterization, 111 did not undergo LVEDP assessment. In-hospital mortality in this group was 9%. Mean LVEF in this group was 36.7±10.0 (versus 52.6±11.6%, p<0.01, in those treated by an invasive strategy without LVEDP measurement and who survived the index admission. These findings highlight the importance of LVEF in predicting in-hospital prognosis during the acute phase of ACS.
Unconventional endpoint and modifiable variablesReadmission for CHF is not a conventional endpoint in most studies on ACS, which generally analyze ischemic outcomes, but it has been investigated by other authors6. The link between HF and coronary disease is well established, as shown by the European Society of Cardiology (ESC) guidelines, which state that ACS and coronary disease are the leading cause of HF in the West11.
With regard to the prescription of diuretics (an indication of fluid overload) within 24 hours of admission, there was no correlation between LVEDP values and pulmonary congestion. This appears counterintuitive, but is in agreement with the findings of other studies on LV filling pressures. For example, in an analysis of the SAVE trial, 89 patients out of 141 (63%) with LVEDP ≥30 mmHg were classified in Killip class I7. On the other hand, another study has reported elevated LVEDP even in patients with no signs of HF, and suggested that this, together with increased heart rate, could be a compensatory mechanism to maintain cardiac output during the acute phase of myocardial infarction12.
In the multivariate analysis model used for the primary endpoint, most variables implicated in readmission for CHF at one year, such as age and LVEDP, do not appear to be modifiable. On the other hand, prescription of beta-blockers had a significant protective effect, reducing relative risk for this outcome by around 70%. The rate of beta-blocker prescription in the study population was 84% within 24 hours of admission, and 80% at discharge. Data in the literature confirm the importance of carvedilol (the most commonly prescribed beta-blocker in our population) in reducing interstitial fibrosis and myocyte hypertrophy, increasing calcium sensitivity and downregulating expression of G proteins in the myocardium13. This not only improves systolic function, but also helps preserve diastolic function, as demonstrated in an echocardiographic study that evaluated the E/A ratio in patients treated with carvedilol14.
Heart failure with preserved left ventricular ejection fractionAccording to the 2007 consensus statement by the ESC, three criteria are required for a diagnosis of heart failure with preserved systolic function: normal or mildly abnormal systolic LV function (LVEF >50%) in the absence of severe LV dilatation, signs or symptoms of fluid overload, and an echocardiographic, laboratory or invasive marker of abnormal LV relaxation or of increased diastolic distensibility or stiffness15.
The LVEF cut-off of 50% proposed in the latest ESC guidelines on heart failure11 replaces the 45% cut-off recommended in 199816. Analysis of the main evidence used as the basis for the guidelines, the CHARM (Candesartan in Heart Failure Reduction in Mortality) study, shows that in terms of prognosis, an LVEF of over 45% loses statistical significance as a predictor of various outcomes, including death, infarction and stroke. In the models proposed that analyzed LVEF in 10% intervals, beginning at 22%, confidence intervals were consistently unity in the group with LVEF above 42%. As pointed out in the guidelines, this means that the 50% criterion is somewhat arbitrary. Moreover, a practice guideline from the Heart Failure Society of America defines preserved systolic function as LVEF of over 40, 45 or 50%17. Given the uncertainty surrounding the best LVEF cut-off to predict prognosis, we opted for 40%, as used in the above-mentioned SAVE trial.
High LVEDP, even with preserved systolic function, is not itself synonymous with HF with normal systolic function, but it is certainly a marker of ventricular or vascular abnormalities related to LV relaxation, which can lead to HF. LVEDP is affected by preload and afterload and is relatively dynamic: revascularization or use of angiotensin-converting enzyme inhibitors or other vasodilators and diuretics can directly influence myocardial distensibility and filling pressures18.
It has been shown that elevated LVEDP in the context of ACS is associated with impaired epicardial and myocardial perfusion6. At the same time, high LVEDP itself affects myocardial perfusion, since it leads to increased vascular resistance and hence impaired coronary microcirculation19.
Various pathophysiological mechanisms may explain the relationship between LVEDP and myocardial distensibility and perfusion. Elevated LVEDP in patients with LVEF ≥40% following ACS may be associated with ischemia, myocardial stunning, myocardial fibrosis, or even changes in coronary microcirculation. We consider that LVEDP should be included in the overall assessment of short- and long-term cardiovascular risk in patients with ACS, given its demonstrated prognostic impact and pathophysiological correlations.
LimitationsAlthough this was a prospective study with retrospective analysis of outcomes, LVEDP was not assessed in 14% of the patients who underwent cardiac catheterization. No other measure of LVEDP was available, and so we could not assess the impact of revascularization or medical therapy during the index admission. A further limitation is the relatively small sample size.
ConclusionElevated LVEDP was a common finding in the acute phase of ACS in this population with LVEF ≥40% treated by an invasive strategy. LVEDP was a significant long-term prognostic marker and an independent predictor of readmission for CHF one year after the index event.
Conflicts of interestThe authors have no conflicts of interest to declare.