With the advent of new oral anticoagulants that do not require regular laboratory control but are significantly more expensive, there has been renewed interest in the quality of the classic agents and the monitoring of patients taking them. We set out to analyze time in therapeutic range of patients under oral anticoagulation monitored in our health unit, to determine whether primary care monitoring is comparable to that in anticoagulation clinics. At the same time, we aimed to ascertain whether there was any association between the dosing method (unit protocol vs. computer-assisted) and the time in therapeutic range achieved.
MethodsWe analyzed all INR values determined in our health unit during the first six months of 2012, using Excel 2007 and SPSS version 17.0, and applying the Student's t test for a level of significance of 0.05.
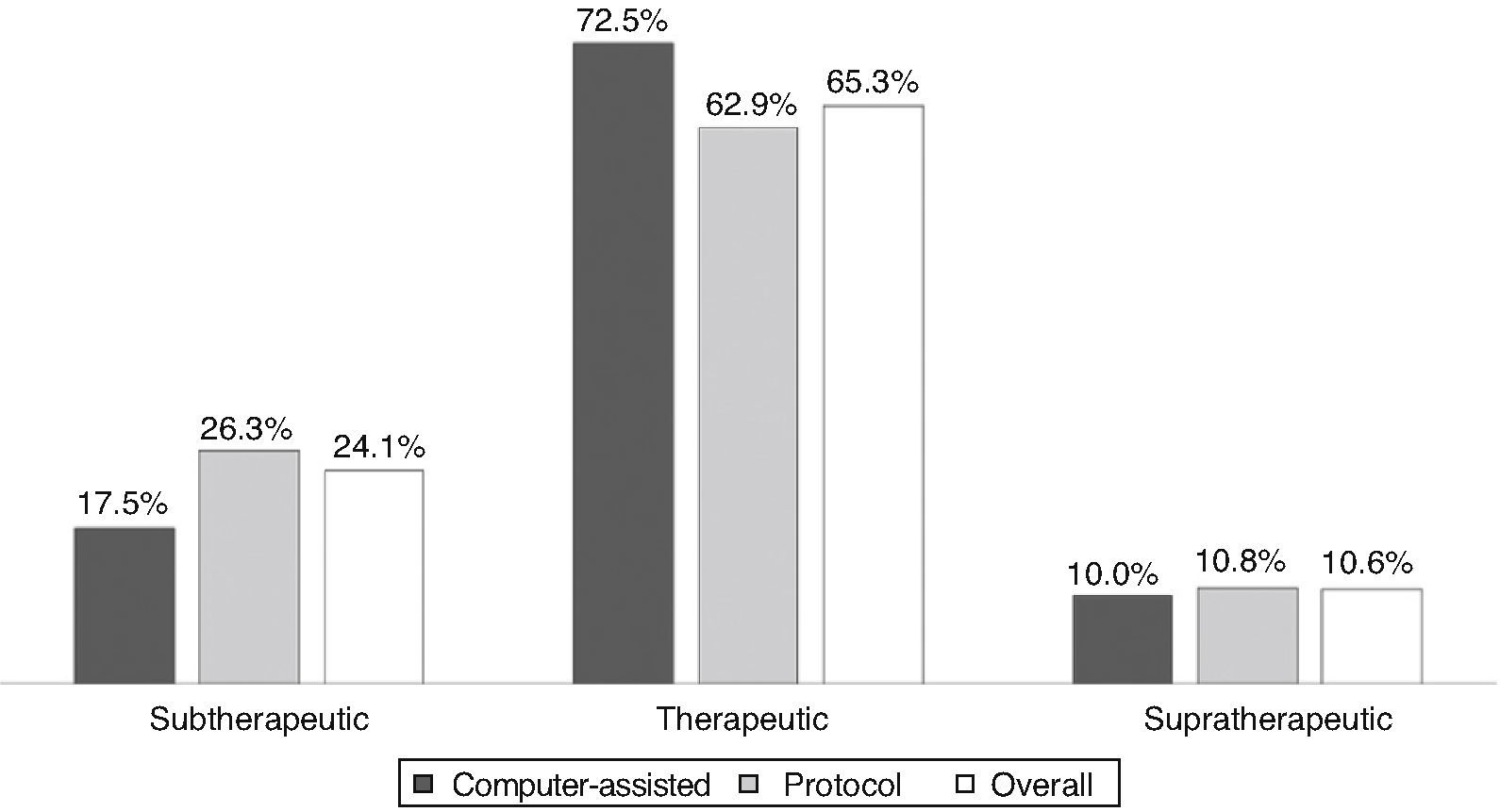
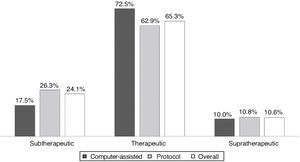
ResultsAll INR assessments during the first six months of 2012 were recorded, a total of 320 tests; mean patient age was 69.9±11.25 years, 63% male. Dose adjustments were made according to the unit protocol in 77% of cases. Atrial fibrillation was the most prevalent indication. Most values (65.3%) were within the target therapeutic range; 24.1% were subtherapeutic and 10.6% supratherapeutic. Computer-assisted dosing achieved better control than the protocol (72.5% vs. 62.9%), without statistical significance.
ConclusionsPrimary care monitoring of oral anticoagulation appears to be comparable to that in anticoagulation clinics, while affording better access and cost reductions.
O aparecimento de novos anticoagulantes orais sem necessidade de controlo laboratorial, mas com custos elevados, relançou o debate sobre a importância da qualidade da anticoagulação oral e do controlo dos doentes. Pretendemos determinar o tempo em intervalo terapêutico dos doentes a fazer anticoagulação oral, seguidos na nossa unidade de cuidados primários, analisando se o controlo em cuidados de saúde primários pode ter qualidade comparável à dos centros hospitalares especializados, e verificar a existência de associação entre o método de ajuste de dose (protocolo do serviço versus programa informático) e o nível de controlo.
MétodosEstudo analítico, com registo de todos os valores de INR determinados na nossa unidade no primeiro semestre de 2012. Programas: Excel 2007, SPSS v. 17.0. Testes: t de Student (n.s. 0,05).
ResultadosObtivemos resultados de 320 testes referentes a 35 doentes, com uma média de idades de 69,9±11,25 anos, sendo 63% do sexo masculino. O ajuste da dose foi feito em 77%, de acordo com o protocolo do serviço. A fibrilhação auricular foi a indicação mais prevalente. A maioria dos testes (65,3%) encontrava-se dentro do intervalo terapêutico; 24,1% apresentaram valores infraterapêuticos e 10,6% supraterapêuticos. O ajuste informático obteve melhor controlo que o protocolo (72,5 versus 62,9%), sem significado estatístico.
ConclusõesA monitorização a nível de cuidados de saúde primários pode ter qualidade igual à das clínicas de anticoagulação, permitindo o controlo mais conveniente dos doentes, dispondo de facilidade de acesso e a diluição dos custos.
Oral anticoagulation (OAC) is used in the treatment and prevention of thromboembolism, the vitamin K antagonists warfarin and acenocoumarol being the most commonly prescribed agents in Portugal. These drugs have a narrow therapeutic window, requiring monitoring and regular dose adjustment to ensure safe and effective levels of anticoagulation.
The advent of new molecules that do not require laboratory control but are significantly more expensive has renewed interest in the classic agents and the quality of INR control. Some authors fear that the results of major clinical trials with these novel agents may have been distorted by comparison with controls in whom the quality of INR control was inadequate.1,2
Although studies3 have shown that these new agents may be more cost-effective, their high direct cost cannot be disregarded since this is currently borne by the patient.
Monitoring of oral anticoagulation therapyThere are three main ways to monitor OAC:
- •
in specialized hospital consultations or anticoagulation clinics;
- •
in primary care centers, usually by the patient's general practitioner, in some cases with computer-assisted dosing;
- •
by patients, using a point-of-care device, either by self-monitoring (patients contact their health center for dose adjustment) or by self-management (patients do the test at home and adjust the dose according to an individualized program).
The most common method in Portugal is hospital monitoring,4 with or without specialized clinics, although primary care monitoring is being implemented in less populated areas of the country with limited access to hospital care, as recommended by the Portuguese National Coordinating Body for Cardiovascular Disease. Patient self-monitoring is growing, albeit slowly, the main obstacle being the price of the device and test strips, which are not currently reimbursed under the National Health System. Another factor is the advanced age and/or low educational level of most patients.
Functions of an anticoagulation clinicAccording to a recent study by Cruz et al.,4 there are three main requirements for the efficient functioning of an anticoagulation clinic:
- •
logistical capacity to treat patients;
- •
technical capacity to perform laboratory tests;
- •
staff trained and experienced in monitoring oral anti-coagulation.
It is important to know whether primary care monitoring can achieve the same quality as specialized hospital units or anticoagulation clinics, since spreading INR monitoring among primary care centers would improve patient access and could result in significant savings. To this end, we present an analysis of our center's experience.
The patients followed in the Santiago family health unit (FHU) have their INR measured on site by a Siemens DCA Vantage® analyzer and are then referred to their general practitioner. Two different dosing methods are used: computer-assisted dosing using the TAOnet® program and dose adjustment according to the Santiago FHU protocol.
The quality of a center's anticoagulation control is assessed by the time in therapeutic range (TTR) of its patients. TTR can be determined in various ways, the most common of which are calculation of the percentage of all INR measurements within the therapeutic range, cross-sectional analysis of medical records to determine the percentage of patients with therapeutic values at a given point in time compared to the total of number of INR measurements at the same point, and application of the Rosendaal linear interpolation method, which assumes a linear relationship between two consecutive INR measurements and allocates a specific INR value to each day between tests.4
ObjectivesWe set out to analyze the TTR of patients under OAC monitored in the Santiago FHU and to ascertain whether there was any association between the dosing method and the TTR achieved.
MethodsThis was a cross-sectional observational study analyzing all INR values determined in the Santiago FHU between January 1 and June 30, 2012 in patients under OAC.
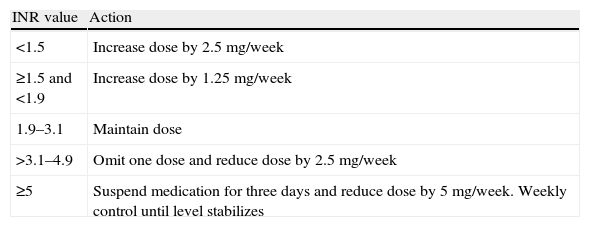
Dose adjustment was performed in one of two ways: computer-assisted dosing using the TAOnet® program, in which the current INR value is entered and the program calculates the dose and time to next control, or according to the unit protocol, shown in Table 1.
Santiago Family Health Unit protocol according to INR value.
| INR value | Action |
| <1.5 | Increase dose by 2.5 mg/week |
| ≥1.5 and <1.9 | Increase dose by 1.25 mg/week |
| 1.9–3.1 | Maintain dose |
| >3.1–4.9 | Omit one dose and reduce dose by 2.5 mg/week |
| ≥5 | Suspend medication for three days and reduce dose by 5 mg/week. Weekly control until level stabilizes |
Patients with active bleeding or INR >7 are referred to the emergency department.
The following characteristics of the study population were analyzed: gender, age, dosing method (protocol vs. computer-assisted), disease necessitating OAC (atrial fibrillation, valve disease, deep vein thrombosis, pulmonary embolism or antiphospholipid syndrome), INR values, and TTR.
TTR was calculated as the percentage of INR measurements within the therapeutic range for each patient (number of INR measurements within the therapeutic range divided by the total number of tests), based on the following values in accordance with national and international guidelines5–7:
- •
subtherapeutic: INR <2.0;
- •
therapeutic: INR ≥2.0–≤3.0;
- •
supratherapeutic: INR >3.0.
Patients with a different target therapeutic range from that defined above were excluded.
Data were recorded and analyzed using Excel 2007. For bivariate analysis, the data were imported into SPSS version 17.0 and the Student's t test was applied, after confirmation of normal distribution of data by the Shapiro-Wilks test and of homogeneity of variance by Levene's test. A level of statistical significance of 0.05 was used.
ResultsWe analyzed all INR values determined during the first six months of 2012, a total of 320 tests in 35 individuals, mean age 69.9±11.25 years (median 73, interquartile range of 17), 63% (n=22) male. The dosing method was chosen by the attending physician and there was no crossover between methods during the study period.
One patient with a mechanical valve prosthesis and a target therapeutic range of 2.5–3.5 was excluded.
Most individuals (n=26, 74%) were under OAC for atrial fibrillation, four (11%) for deep vein thrombosis, two (6%) for pulmonary embolism, two (6%) for valve disease and one (3%) for antiphospholipid syndrome.
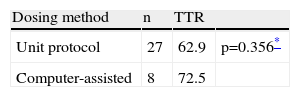
Dose adjustments were made according to the unit protocol in most cases (77%), corresponding to 240 tests, while computed-assisted dosing was performed in eight patients (23%), corresponding to 80 tests (Table 2).
Time in therapeutic range according to dosing method.
| Dosing method | n | TTR | |
| Unit protocol | 27 | 62.9 | p=0.356* |
| Computer-assisted | 8 | 72.5 |
INR values ranged between 1.1 and 5.0 in the 320 tests, of which 209 (65.3%) were within the target therapeutic range, 77 (24.1%) were subtherapeutic (48.1%<1.5), and 10.6% were supratherapeutic.
Figure 1 compares TTR for the three ranges under analysis, overall and according to dosing method. TTR was better in the patient group with computer-assisted dosing, but without statistical significance.
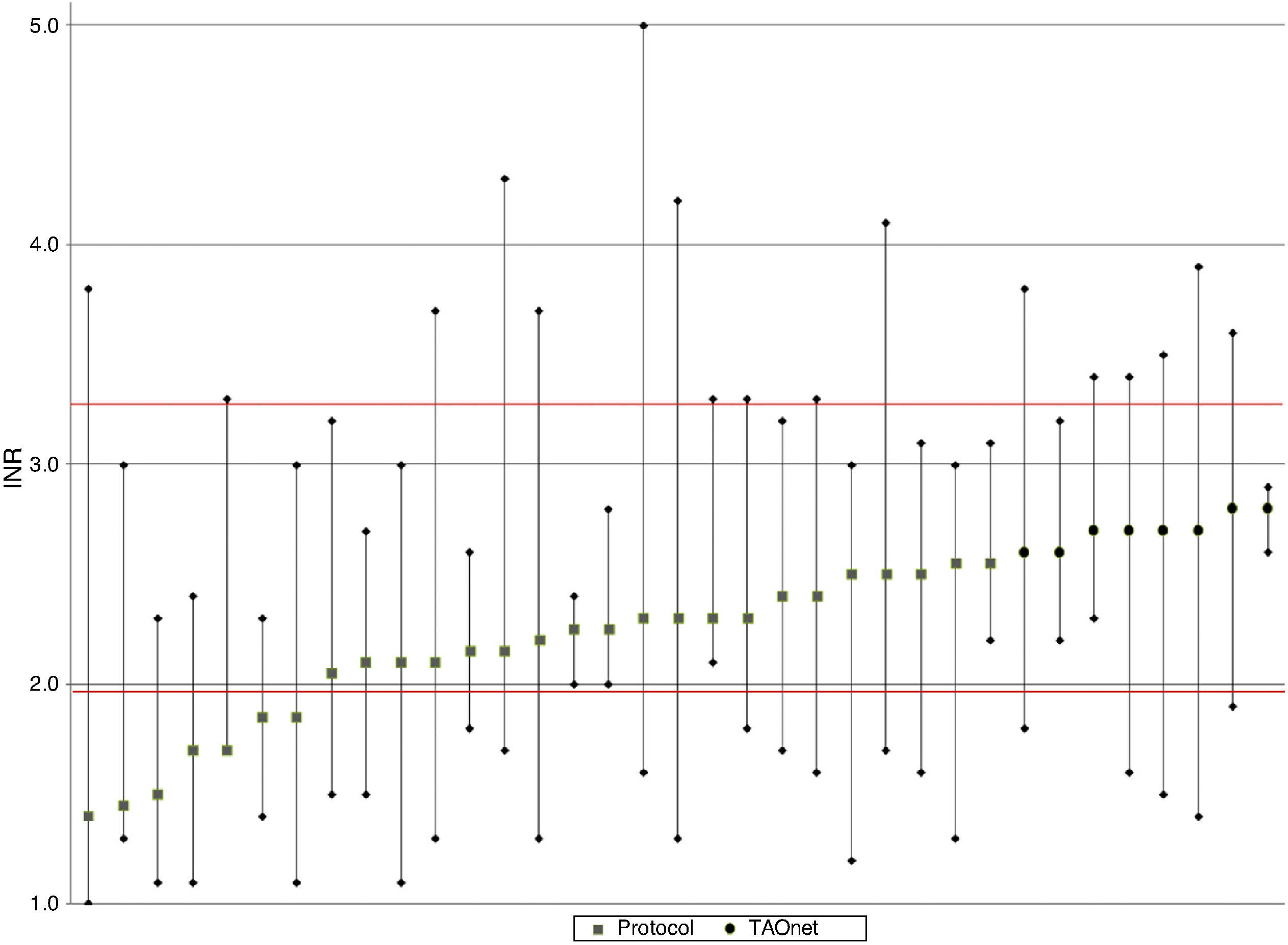
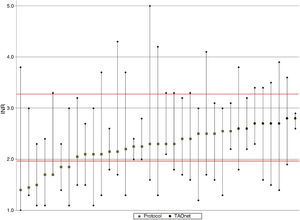
Figure 2 shows mean, maximum and minimum values per patient. There was a greater tendency for better TTR and less variation in the group with computer-assisted dosing (white circles), as well as lower mean INR and greater variation in the group with dose adjustment according to the protocol.
DiscussionTTR in patients under OAC followed in the Santiago FHU was 65.3%. Although TTR was greater and there was less INR variation in patients with computer-assisted dosing, there was no statistically significant association between dosing method and level of INR control.
These results are in agreement with the latest Portuguese studies in this area, and are similar to those achieved in anticoagulation clinics and other primary care centers and higher than those found in a meta-analysis8 of 67 studies, which reported a TTR of only 57% for primary care monitoring.
Computer-assisted dosing resulted in greater TTR and less INR variation, as well as a lower frequency of extreme, particularly subtherapeutic, INR values, as also reported in the literature.
The therapeutic range was defined as INR 2.0–3.0, in accordance with international guidelines, and the cut-offs for dose adjustment were therefore ≤1.9 and ≥3.1. However, according to Rose et al.,9 cut-offs of 1.7 and 3.3 are more appropriate since this avoids excessive dose adjustments which tend to destabilize INR and results in greater TTR.
One obvious limitation of the present study is the small sample size, which would have contributed to the lack of statistical significance in its results.
The authors intend to implement certain improvements in the future, particularly making computer-assisted dosing the preferred method of dose adjustment. They also plan to extend the study to other centers in order to increase the population base, followed by a re-evaluation.
In our experience, computer-assisted dosing appears to facilitate the task of dose adjustment, as well as reducing risk to the patient.10,11 It also enables dose adjustment to be performed by non-physicians, such as home monitoring by nursing teams.
Some authors fear that the results obtained in major trials with new anticoagulants may have been distorted by comparison with controls in whom the quality of INR control was inadequate. Our results indicate that it would be difficult to obtain similar TTR rates to those reported in these trials, since highly specialized centers would be required, which would inevitably lead to increased indirect costs and concentration of services.
Although most studies report greater efficacy and safety with the new oral anticoagulants, their high cost could be a major barrier to their widespread use, especially since the number of patients under OAC is predicted to rise. Rigorous cost-effectiveness studies are required to dispel doubts concerning the real savings to be gained with these new drugs. Until then, the classic drugs will continue to be used in everyday practice, and hence the quality of anticoagulation control remains just as relevant.
ConclusionsPrimary care monitoring of OAC appears to be comparable to that in anticoagulation clinics, is more convenient for patients, and may result in savings through improved patient access and cost reductions in staff and equipment.
The authors are not advocating either classic or novel drugs; while the new agents do not require laboratory control, they entail higher direct costs, which are not currently reimbursed under the National Health Service, with the exception of dabigatran 110 mg, which has not been shown to be superior to warfarin.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Agnelo P, Alexandra D, Matias S. Monitorização de doentes sob anticoagulação oral numa unidade de cuidados de saúde primários. Rev Port Cardiol. 2014;33:397–401.