Mitral regurgitation (MR) is the most common valvular disease and has recently been the target of a number of percutaneous approaches. The MitraClip is virtually the only device for which there is considerable experience, with more than 20000 procedures performed worldwide.
ObjectiveTo describe our initial experience of the percutaneous treatment of MR with the MitraClip device.
MethodsWe describe the first six MitraClip cases performed in this institution (mean age 58.5±13.1 years), with functional MR grade 4+ and New York Heart Association (NYHA) heart failure class III or IV (n=3), with a mean follow-up of 290±145 days.
ResultsProcedural success (MR ≤2+) was 100%. Total procedure time was 115.8±23.7 min, with no in-hospital adverse events and discharge between the fourth and eighth day, and consistent improvement in the six-minute walk test (329.8±98.42 vs. 385.33±106.95 m) and in NYHA class (three patients improved by two NYHA classes). During follow-up there were two deaths, in two of the four patients who had been initially considered for heart transplantation.
ConclusionIn patients with functional MR the MitraClip procedure is safe, with both a high implantation and immediate in-hospital success rate. A longer follow-up suggests that the clinical benefit decreases or disappears completely in patients with more advanced heart disease, namely those denied transplantation or on the heart transplant waiting list.
A insuficiência valvular mitral (IM) é a valvulopatia mais comum e começa agora a ser alvo de abordagens percutâneas. O dispositivo MitraClip é virtualmente o único dispositivo com uma experiência considerável, ultrapassando atualmente as 20000 implantações.
ObjetivoDescrever a experiência inicial da terapêutica percutânea da IM com o dispositivo MitraClip.
MétodosDescrevem-se os primeiros seis casos de MitraClip realizados nesta instituição (idade 58,5±13,1 anos), com IM 4+ de etiologia funcional e insuficiência cardíaca classe III ou IV (n=3) da New York Heart Association (NYHA), sem indicação cirúrgica ou recusados para cirurgia, com um follow-up médio 290±145 dias avaliado clinicamente pela sobrevivência, classe funcional, teste da marcha dos seis minutos, estudo ecocardiográfico e determinação do proBNP.
ResultadosO sucesso do procedimento (IM ≤2+) foi de 100%, com um tempo de procedimento de 115,8±23,7mins, sem intercorrências intra-hospitalares, alta entre o 4.° e 8.° dia e uma melhoria consistente no teste da marcha dos seis minutos (329,8±98,42 versus 385,3±106,95m) e da classe funcional (três dos pacientes com melhoria de duas classes funcionais NYHA). Na totalidade do follow-up (290±145 dias) constatam-se dois óbitos, em dois dos quatro pacientes que tinham sido considerados para transplantação cardíaca.
ConclusõesO procedimento de implantação do dispositivo MitraClip é seguro, com uma alta taxa de sucesso imediato nos pacientes com IM funcional. A médio prazo o benefício clínico parece esbater-se ou anular-se nos pacientes com insuficiência cardíaca mais terminal, nomeadamente nos pacientes a aguardar ou recusados para transplante.
Mitral regurgitation (MR), of varying severity, is the most common valvular disorder, with a prevalence of 1.7%, increasing to 10% in those aged over 75.1 However, around half of patients with severe MR are not referred for surgery, mainly due to advanced age, comorbidities or severe left ventricular dysfunction.2 Of those who do undergo surgery, only 34–53%3 are treated by valvuloplasty rather than valve replacement.
There are two types of MR: anatomical, in which one of the elements of the valve apparatus (annulus, leaflets or chordae) is dysfunctional; and functional, in which there is a geometric and/or functional alteration in the left ventricle that changes the normal relationship between these elements.
Sooner or later, severe MI leads to left ventricular dysfunction and hence congestive heart failure. Although medical therapy can relieve symptoms, it does not alter progression of the disease, which is responsible for annual mortality of ≥5% in symptomatic individuals.4,5 Another reason for the large number of patients with severe MR who are not referred for surgery is that its benefit in functional MR is unproven. Although functional MR is 5–10 times more prevalent than anatomical MR or aortic stenosis,6 surgical treatment has only a class IIb recommendation in the European and American guidelines for chronic secondary MR unless coronary artery bypass grafting is also indicated.7 Percutaneous procedures thus do not in fact compete with surgery for the treatment of functional MR.
Treatment of MR was until recently exclusively surgical, but in recent years a number of percutaneous approaches have been developed, of which the most widely used with consistent results involves implantation of a clip device, the MitraClip (Abbott Laboratories, Abbott Park, IL, USA).
There have been various approaches for the treatment of MR:
- •
Annulus: this D-shaped orifice, which is in fact the left atrioventricular junction,1 is nonplanar, with elevated septal and lateral segments. The goal of surgical annuloplasty is to decrease the septal-to-lateral diameter by at least 8 mm.8
- •
Leaflets: the anterior leaflet is in fibrous continuity with the coronary and non-coronary leaflets of the aortic valve, and has a wider surface with a shorter base, guarding only one-third of the left atrioventricular junction, than the posterior leaflet. The surface area of both leaflets taken together is 2.5 times the area of the valvular orifice. In systole, the leaflets coapt over a height of, on average, 8 mm, giving a “coaptation reserve” in case of annular dilation.1
- •
Chordae tendineae: these connect the valve leaflets to the ventricular free wall via the papillary muscles or directly to the wall (basal cords).
- •
Ventricular geometry: changes in left ventricular geometry, whether due to ischemia or other processes, can severely disrupt the dynamics of mitral coaptation.9 Dyskinesia can alter the orientation of the basal cords, leading to tethering of the posterior leaflet, and ventricular dilatation can cause the papillary muscles to move apically, leading to leaflet tenting and loss of coaptation.
The Alfieri stitch is a surgical treatment for severe MR due to incomplete leaflet coaptation that consists of suturing together the free edges of the middle segments of the anterior and posterior leaflets (A2 and P2 scallops), together with annuloplasty,10 and configuring the valve with a double orifice. However, the group who developed this procedure discovered that omitting the annuloplasty often produced a good hemodynamic result, which was the rationale for a percutaneous approach with the MitraClip.11
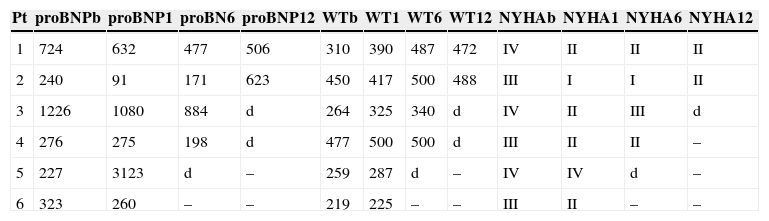
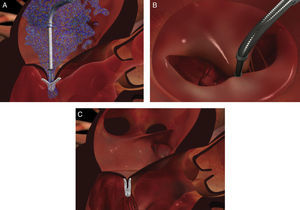
MethodsOf a total of 20 patients referred for this procedure over a two-year period, six were initially selected for the treatment after transthoracic and transesophageal echocardiographic assessment. Clinical criteria were functional MR grade 4+, New York Heart Association (NYHA) class III or IV and previous admissions for heart failure, and they also had to have exhausted all other treatment options, surgical or otherwise, including cardiac resynchronization therapy. Most of those referred were being followed in heart failure clinics. Patients who fulfilled the clinical criteria also had to meet the anatomical criteria (Figure 1) for implantation of the MitraClip device. As the technique has become more widespread, the initially proposed anatomical criteria have been somewhat modified, but they should be closely followed by centers beginning to use the procedure. The criteria are: central MR jet; coaptation length >2 mm and coaptation depth <11 mm; in the event of a flail leaflet, flail gap and flail width should be no more than 10 and 15 mm, respectively.
These criteria inevitably exclude patients with severe left ventricular dilatation, since dilatation of the annulus separates the leaflets to such an extent that it is extremely difficult to implant the clip. All echocardiographic records were reviewed by a proctor, who decided on the feasibility of the procedure.
In demographic terms (Table 1) this initial experience involved relatively young patients (mean age 58.5±13.1 years), with a mean ejection fraction (EF) of 29±10.36% and logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) of 20.06±16.86. Four patients had been assessed for heart transplantation, one of whom had been rejected due to fixed pulmonary vascular resistance. Three patients would have been excluded from the Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) trial12 due to EF <25% and/or left ventricular end-systolic dimension of >55 mm (mean 55.0±7.19 mm).
Demographic and clinical characteristics of the study population.
| Patient | Gender | Age (years) | EF (%) | EVER II | LVESD | log ES | ES II | Func | Org | MR | NYHA | Etiology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | 24 | N | 65 | 12.04 | 3.24 | Y | N | 4 | IV | Ischemic |
| 2 | M | 62 | 45 | S | 48 | 10.73 | 1.64 | Y | N | 4 | III | Myocarditis |
| 3 | F | 48 | 22 | N | 59 | 12.04 | 4.50 | Y | N | 4 | IV | Dilated |
| 4 | M | 49 | 33 | S | 45 | 8.96 | 2.1 | Y | N | 4 | III | Dilated |
| 5 | M | 52 | 26 | N | 61 | 19.58 | 2.3 | Y | N | 4 | IV | Dilated |
| 6 | M | 83 | 38 | S | 52 | 57.03 | 9.5 | Y | N | 4 | III | Ischemic |
EF: ejection fraction; ES II: EuroSCORE II; EVER II: EVEREST II criteria; F: female; Func: functional MR; log ES: logistic EuroSCORE; LVESD: left ventricular end-diastolic diameter in mm; M: male; MR: mitral regurgitation grade; N: no; NYHA: New York Heart Association class; Org: organic or degenerative MR; Y: yes.
The selected patients underwent transthoracic echocardiography before the procedure, at discharge, and at one, six and 12 months, as well as clinical assessment, pro-brain-type natriuretic peptide (proBNP) measurement, and six-minute walk test the day before implantation and at one, six and 12 months post-implantation. All patients gave their written informed consent.
Procedural success was defined as grade 2+ or less MR in the absence of complications.12
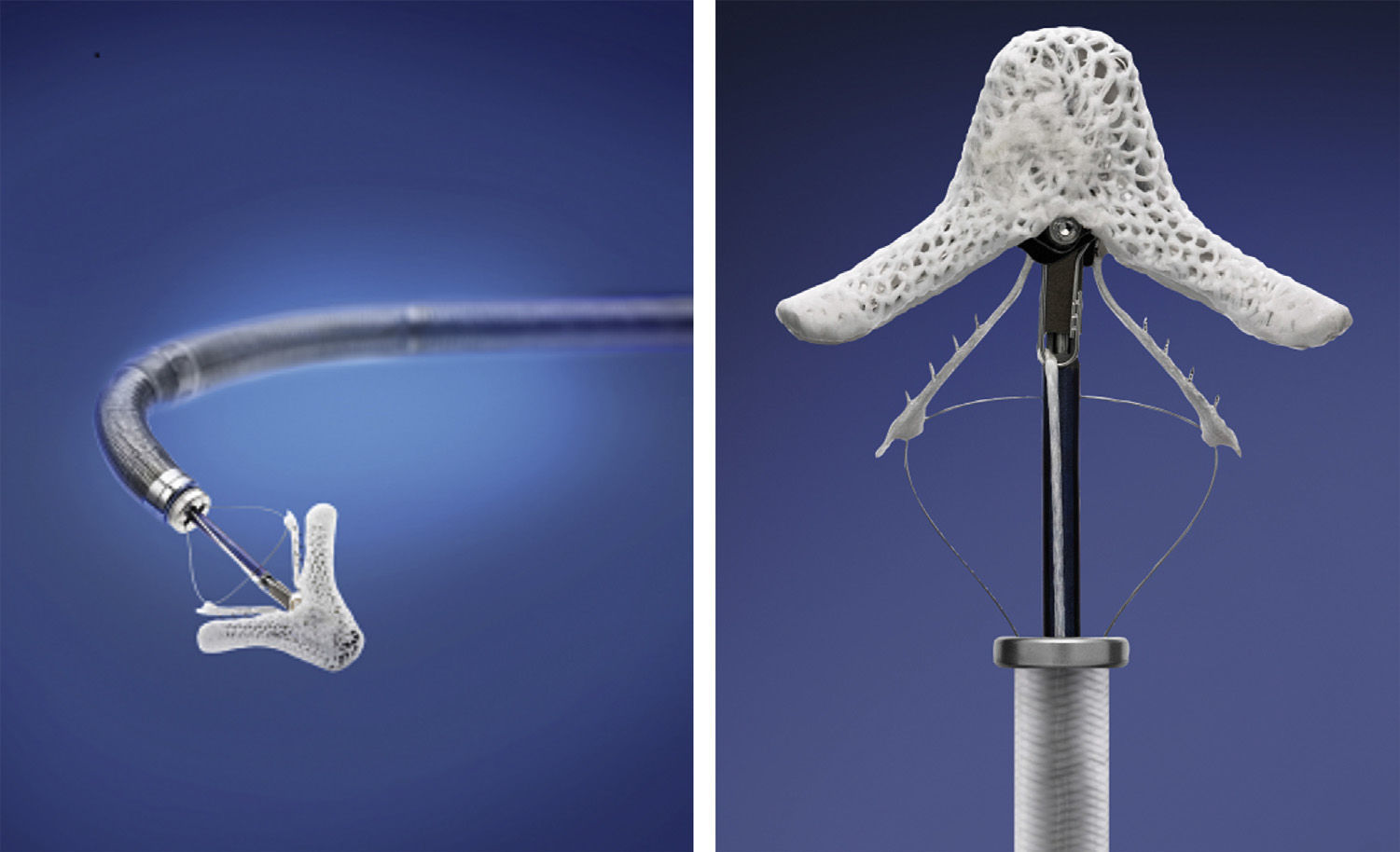
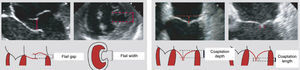
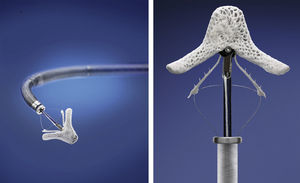
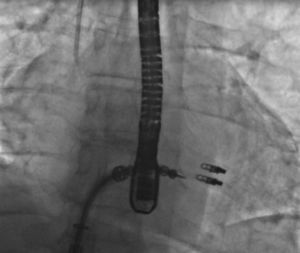
The MitraClip resembles a clothes-peg, and is made of cobalt chromium, 4 mm wide, with two articulated arms that open from 0° (closed position) to 240° (open position), and uses a delivery system (Figures 2 and 3) that enables the arms to be opened and closed and the device to be maneuvered in three dimensions (rotated and moved in anterior, posterior, lateral and medial directions).
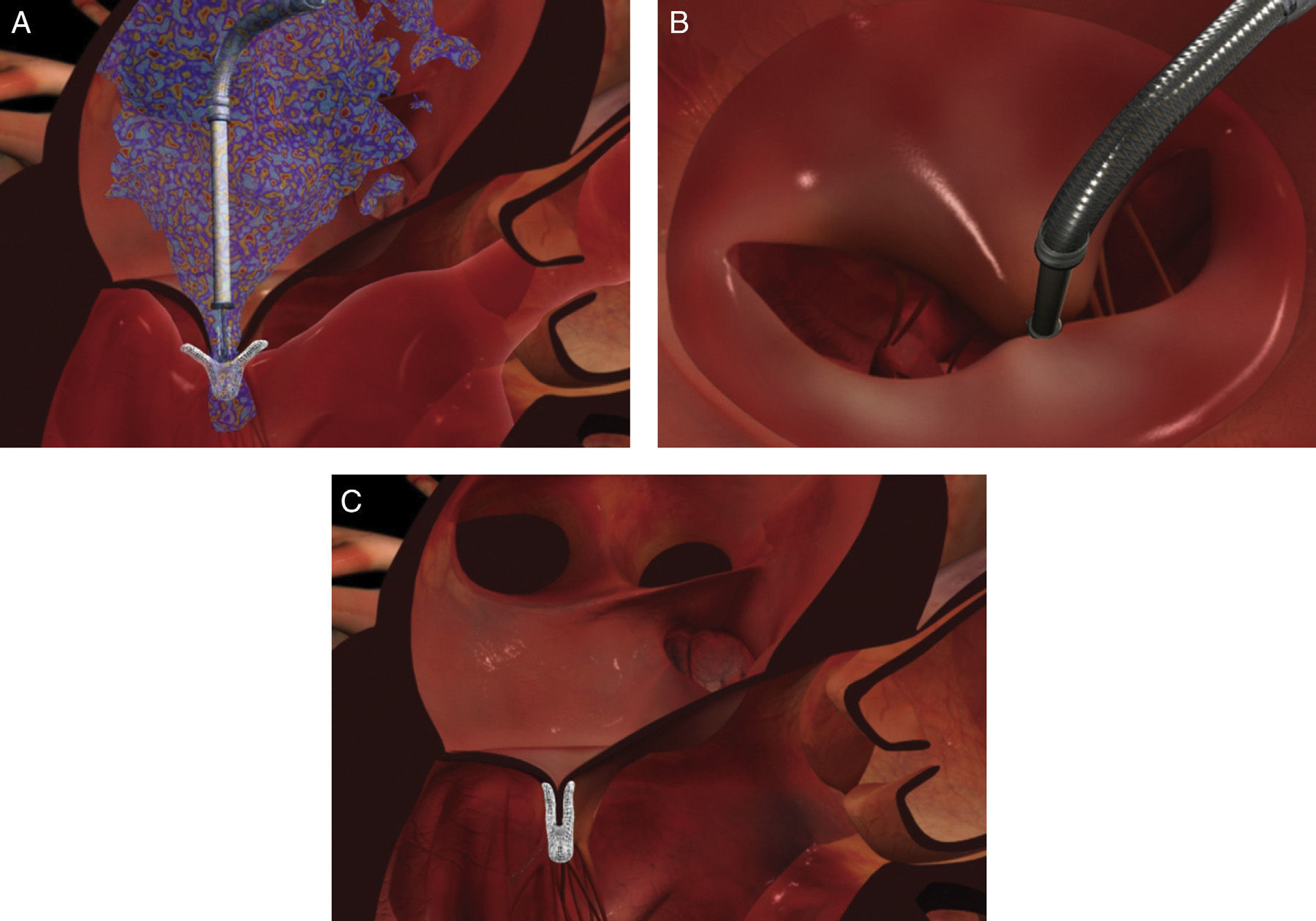
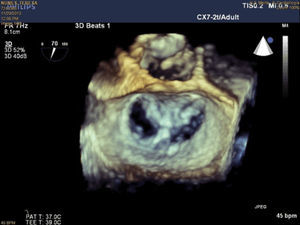
The procedure is performed under general anesthesia. Via a femoral approach, a 24F delivery system is introduced into the left atrium following transseptal puncture, under fluoroscopic and transesophageal echocardiographic guidance. Three-dimensional echocardiography is essential, since it allows simultaneous visualization in two orthogonal planes (x-plane mode) and thus helps to fine-tune the delivery and placement of the device and fixation of the leaflets. The clip is moved to the base of the regurgitant flow and grasps the mid segments of both leaflets, bringing them together, and is only released when the jet is deemed to be sufficiently reduced (Figure 4). If the leaflets cannot be correctly fixed, the arms can be opened to release the leaflets, and the clip withdrawn to the left atrium to be repositioned. Between one and four clips are usually implanted. Following implantation, a fibrous bridge forms between the two leaflets, providing additional strength and reducing the risk of septal-lateral distension.
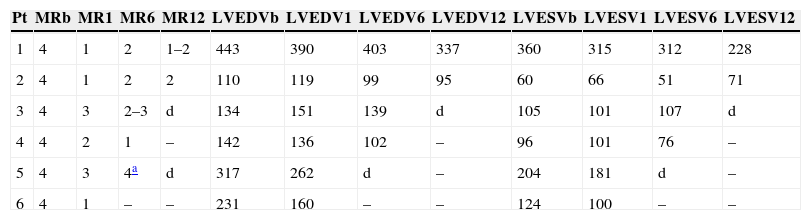
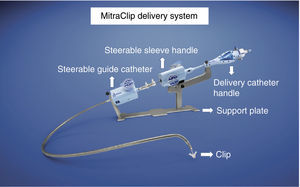
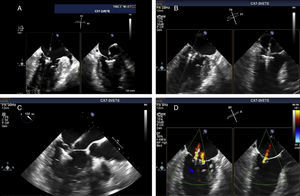
The procedures were performed in the catheterization laboratory of the cardiology department of Hospital de Santa Marta, Lisbon, under general anesthesia and two- and three-dimensional transesophageal echocardiographic guidance (Figure 5). Three proctors (an interventional cardiologist, an echocardiographic cardiologist and a device technician) were present for the first two cases and one, a device technician, was present for the others. Following transseptal puncture and administration of intravenous heparin to achieve an activated clotting time >250 s, the 22F MitraClip delivery system was positioned in the left atrium via a 24F sheath in the right femoral vein. One MitraClip was placed at the base of the regurgitant jet, dividing it into two; if these jets were small, the clip was implanted, otherwise two clips were used (Figure 6), implanting the first in a mid position and the second more laterally (Figure 7), which was only released after confirmation that there was no significant transmitral gradient. If the leaflets were particularly difficult to grasp, a brief period of apnea was induced by disconnecting the patient from the ventilator for a few seconds and/or intense bradycardia was induced by administering a short half-life beta-blocker (esmolol).
Implantation of a second MitraClip. If it is expected that a second clip will be required, the first should be placed in a mid position and the second more laterally. (A and B) Confirmation in different views of the position of the MitraClip; (C) the two mitral valve leaflets are grasped by the MitraClip; (D) after release, with residual regurgitation jets.
In accordance with our protocol, patients were extubated in the catheterization laboratory and transferred already conscious to the intensive care unit (ICU) for 24 hours, after which they were transferred to the ward.
Since five patients were under oral anticoagulation with acenocoumarol or warfarin, clopidogrel was prescribed for a month following the procedure only in the remaining patient, who was taking aspirin.
Results of the statistical analysis of quantitative variables in these six cases were expressed as means ± standard deviation.
ResultsProcedural success in these six patients was 100%, with no adverse events during the intervention or during hospital stay (mean 6.2±1.48 days).
Although the intervention is relatively lengthy (115.8±23.7 min), fluoroscopy time is relatively short (49.2±19.38 min), because the procedure is mainly guided by transesophageal echocardiography (Table 2).
Procedural data and hospital stay.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | Mean ± SD |
|---|---|---|---|---|---|---|---|
| Fluoroscopy time (min) | 41 | 73 | 35 | 26 | 78 | 42 | 49.2±19.38 |
| Procedure time (min) | 80 | 155 | 110 | 105 | 135 | 110 | 115.8±23.7 |
| Device time (min) | 60 | 130 | 85 | 85 | 100 | 90 | 91.7±20.9 |
| Radiation dose (mGy) | 915 | 2680 | 1487 | 209 | 4456 | 2026 | 1962.2±1362.3 |
| No. of clips implanted | 1 | 2 | 1 | 1 | 2 | 1 | 1.3±0.47 |
| Hospital stay after procedure (days) | 6 | 6 | 7 | 4 | 8 | 5 | 6.0±1.29 |
Device time: time between entry of the steerable guide catheter into the left atrium via the interatrial septum and withdrawal of the MitraClip delivery catheter into the steerable guide catheter; Procedure time: time between transseptal puncture and removal of the steerable guide catheter.
Echocardiographic assessment (Tables 3 and 4) revealed improvement in left ventricular end-diastolic (229.5±118.53 vs. 182.5±88.24 ml) and end-systolic (158.2±100.28 vs. 127.2±57.71 ml) volume, EF (31.3±8.2 vs. 37.3±6.11%) and pulmonary artery systolic pressure (44.5±16.75 vs. 41.0±9.97 mmHg).
Echocardiographic evolution of mitral regurgitation and ventricular volumes.
| Pt | MRb | MR1 | MR6 | MR12 | LVEDVb | LVEDV1 | LVEDV6 | LVEDV12 | LVESVb | LVESV1 | LVESV6 | LVESV12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 1 | 2 | 1–2 | 443 | 390 | 403 | 337 | 360 | 315 | 312 | 228 |
| 2 | 4 | 1 | 2 | 2 | 110 | 119 | 99 | 95 | 60 | 66 | 51 | 71 |
| 3 | 4 | 3 | 2–3 | d | 134 | 151 | 139 | d | 105 | 101 | 107 | d |
| 4 | 4 | 2 | 1 | – | 142 | 136 | 102 | – | 96 | 101 | 76 | – |
| 5 | 4 | 3 | 4a | d | 317 | 262 | d | – | 204 | 181 | d | – |
| 6 | 4 | 1 | – | – | 231 | 160 | – | – | 124 | 100 | – | – |
b, 1, 6, 12: baseline, 1 month, 6 months, 12 months, respectively; d: died; LVEDV: left ventricular end-diastolic volume in ml; LVESV: left ventricular end-systolic volume in ml; MR: mitral regurgitation grade; Pt: patient.
Echocardiographic evolution of ejection fraction and pulmonary artery systolic pressure.
| Patient | EFb | EF1 | EF6 | EF12 | PASPb | PASP1 | PASP6 | PASP12 |
|---|---|---|---|---|---|---|---|---|
| 1 | 24 | 17 | 23 | 33 | 33 | 33 | 38 | 33 |
| 2 | 45 | 44 | 44 | 25 | 24 | 35 | 32 | 42 |
| 3 | 22 | 33 | 23 | d | 78 | 58 | 62 | d |
| 4 | 33 | 26 | 25 | – | 42 | 57 | 36 | – |
| 5 | 26 | 19 | d | – | 44 | 33 | d | – |
| 6 | 38 | 37 | – | – | 46 | 40 | – | – |
b, 1, 6, 12: baseline, 1 month, 6 months, 12 months, respectively; d: died; EF: ejection fraction (%); PASP: pulmonary artery systolic pressure (mmHg).
At one-month follow-up (Table 5), functional status had improved in five of the six patients, with an improvement of one (two patients) or two (three patients) NYHA classes. There were a total of three readmissions (two in one patient) and two deaths, in two of the four patients who had been initially considered for heart transplantation. Of these, one had been rejected due to fixed pulmonary vascular resistance and the other was in terminal heart failure before the MitraClip procedure. This patient was readmitted a month later for congestive heart failure and died 84 days after admission without a donor having been found; he was the only patient in whom proBNP had risen significantly at one-month follow-up, all the others showing a fall in this parameter (557.8±376.73 vs. 494.2±249.5 ng/l). The patient who had been denied transplantation was briefly admitted twice within six months of MitraClip implantation (before the procedure she had been admitted virtually every month) and suffered outpatient sudden death after nine months.
Clinical and laboratory follow-up.
| Pt | proBNPb | proBNP1 | proBN6 | proBNP12 | WTb | WT1 | WT6 | WT12 | NYHAb | NYHA1 | NYHA6 | NYHA12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 724 | 632 | 477 | 506 | 310 | 390 | 487 | 472 | IV | II | II | II |
| 2 | 240 | 91 | 171 | 623 | 450 | 417 | 500 | 488 | III | I | I | II |
| 3 | 1226 | 1080 | 884 | d | 264 | 325 | 340 | d | IV | II | III | d |
| 4 | 276 | 275 | 198 | d | 477 | 500 | 500 | d | III | II | II | – |
| 5 | 227 | 3123 | d | – | 259 | 287 | d | – | IV | IV | d | – |
| 6 | 323 | 260 | – | – | 219 | 225 | – | – | III | II | – | – |
b, 1, 6, 12: baseline, 1 month, 6 months, 12 months, respectively; d: died; NYHA: New York Heart Association class; proBNP: pro-brain-type natriuretic peptide (ng/l); WT: six-minute walk test (m).
The one-month six-minute walk test showed an improvement in all patients (329.8±98.42 vs. 385.33±109.95 m). The four patients still alive at the end of follow-up (290±140 days) maintained an improvement of one or two NYHA functional classes (Table 5).
DiscussionThe six cases described constitute our initial experience with the MitraClip. This small number, together with a still short follow-up and the fact that the echocardiographic studies were not evaluated by an independent laboratory, are the main limitations of this study and prevent any generalizations. Nevertheless, the most striking finding is the safety of the intervention.
We began using this procedure after a two-year period of patient selection, which included detailed study of the mitral anatomy by transthoracic and transesophageal echocardiography. There are currently eight patients on the waiting list, all with severe MR and symptoms of heart failure. Accumulated experience worldwide suggests that when a center first begins to use the device only one in three patients referred for MitraClip implantation is considered suitable.
The device received CE Mark approval in 2008 and US Food and Drug Administration approval in 2013, the latter on the basis of the EVEREST II trial, in which 279 patients with MR grade 3+ or 4+ were randomized in a 2:1 ratio to MitraClip or surgery (repair or replacement). The primary composite endpoint for efficacy was freedom from death, from surgery for mitral valve dysfunction, and from grade 3+ or 4+ MR at 12 months, and the primary safety endpoint was a composite of major adverse events within 30 days. The rates of the primary end point for efficacy were 55% in the MitraClip group and 73% in the surgery group (p=0.007), with 6% mortality in each group, 20% vs. 2% surgery for mitral valve dysfunction, and 21% vs. 20% grade 3+ or 4+ MR. The rates for the safety endpoint were 15% vs. 48% (p<0.001). At 12 months, both groups had improved left ventricular size, NYHA functional class, and quality-of-life measures.
Of the patients treated with the MitraClip, 41 (23%) had grade 3+ or 4+ MR before hospital discharge and were referred for surgery. Of these 41 patients, 28 underwent subsequent mitral valve surgery. In the surgery group, mitral valve replacement was performed in 14% and repair in 86%.
Transfusion of ≥2 units of blood (which has an important effect on late outcomes13) comprised the largest single component of major adverse events at 30 days. Even after the exclusion of transfusion events, the rate of adverse events was lower in the MitraClip group.
In two subgroups in the MitraClip arm, those with functional MR and patients aged ≥70 years, efficacy was not inferior to surgery. Four-year results in the EVEREST II trial show 83% survival in the MitraClip group and 82% in the surgical group, and no significant difference in the number of patients with MR grade 3+ or 4+.14 The efficacy and safety of the device compared with medical therapy in high-risk patients has been confirmed by the European ACCESS-EU registry15 and the German TRAnscatheter Mitral valve Interventions (TRAMI) registry.16 Also comparing the MitraClip with standard of care are the randomized trials Clinical Outcomes Assessment of the MitraClip Percutaneous Therapy for Extremely High Surgical Risk Patients (COAPT) in the USA and A Randomized Study of the MitraClip Device in Heart Failure Patients with Clinically Significant Functional Mitral Regurgitation (RESHAPE-HF) in Europe.
The most striking demographic feature in our series was the relatively young age of the patients (only one octogenarian; mean age 58.5±13.1 years), which was the main reason for the low logistic EuroSCORE of 20.06±16.86. Given the weight attributed to age in this risk score, the real risk of these patients may have been underestimated, as indicated by the two non-procedure-related deaths in the 12 months after implantation. The purpose of selecting such young patients was to use the device in those with the longest expected survival, who would thus obtain the maximum possible benefit from the procedure. The idea of choosing patients who were under consideration for heart transplantation was to postpone for as long as possible – ideally for years – the need to go on the waiting list for a donor. However, the results suggest that in some of these patients, the natural history of the disease has progressed too far for them to derive any benefit. Patient selection remains the most important factor, since the purpose of the MitraClip is to improve symptoms, but current evidence indicates that a quarter of patients experience little or no symptomatic relief.
Other series of patients mainly with functional MR,17 including the largest European registry and the one that is perhaps closest to our experience, ACCESS-EU,15 show that 72–83% of patients who survive to one-year follow-up have grade ≤2+ MR and 79–83% are in NYHA class I or II. Hospital stay in ACCESS-EU was slightly longer than in our series (7.7±8.2 days, including 2.5±6.5 days in the ICU), and as in our series there was consistent improvement in echocardiographic parameters and in the six-minute walk test; in the latter the mean gain was around 60 m, also similar to our experience (55 m). Hospital stay in European series tends to be longer than in US registries, in which the average is three days.
Historically, US and European approaches have differed. EVEREST I18 and II were American trials, and they gave initial credibility to the MitraClip device; their patients also had to be candidates for surgery. In Europe, by contrast, the focus has been more on patients who would be ineligible for such trials: those with severe functional MR and heart failure, not considered suitable for surgery and often non-responders to cardiac resynchronization therapy, most of whom are being followed in heart failure clinics and some under consideration for or denied transplantation, as were four of our patients. In Europe MitraClip implantation is perceived as a procedure that does not compete with surgery for patients who have no non-pharmacological alternative. Thus, unlike in EVEREST II, in which 73% of the trial population had degenerative MR, in our series all six patients had functional MR. As a result, unlike transcatheter aortic valve replacement, there is little possibility of a turf war between interventional cardiology and cardiac surgery.
There are two patient groups for whom MitraClip therapy is particularly attractive: those with severe annular calcification, since this can act as a prosthetic ring to provide additional support and thus prevent progressive annular dilatation; and those with severe functional MR and heart failure refractory to pharmacological and resynchronization therapy (non-responders).19
Despite the successful and increasing use of the procedure, the MitraClip does have some potential limitations.20 It is not a complete substitute for the Alfieri stitch, since it does not treat the annulus, which may explain the recurrence of significant MR in the medium to long term; it can be difficult to quantify MR, particularly residual regurgitation after implantation of the clip during a procedure under general anesthesia (although this problem is also found with surgery); MR could be replaced by mitral stenosis, although no cases have been reported to date21; and there are no data on its long-term durability and efficacy.
Meanwhile, as the procedure has become more widespread, the initial anatomical criteria have been widened, with cases reported of treatment of eccentric MR, and variations on the original technique have been developed, such as the zipping technique, in which four or five clips are implanted.
There are a growing number of percutaneous MR therapies besides the MitraClip, some of them already being implemented. These can be divided into two main types, according to whether they involve the annulus or other elements of the mitral valve apparatus.
In most surgical approaches to MR, the two main components of the valve, the leaflets and the annulus, are treated. It is thus understandable that there has also been extensive research into percutaneous interventions on the annulus, which in fact precedes the development of the MitraClip.
There are two forms of percutaneous annuloplasty, indirect and direct. The CARILLON Mitral Contour System (Cardiac Dimensions) is an indirect annuloplasty device consisting of two nitinol anchors connected by a central nitinol element, positioned in the coronary sinus. It is delivered via a 9F catheter through the internal jugular vein, and tension on the device is adjusted manually by moving it 4–5 cm before final release. The procedure is monitored by transesophageal echocardiography, to assess MR grade, and left coronary angiography, to exclude coronary compression. It is the only percutaneous annuloplasty device currently approved in the European Union.
Unlike indirect annuloplasty devices, which are positioned in the coronary sinus, direct approaches treat the annulus itself, as in surgical repair. Access is more complex with direct devices. With the Mitralign Bident system, the device is introduced retrogradely via the femoral artery and positioned in the left ventricle under the posterior leaflet. Two pledget pairs are implanted in the annulus and plicated with a Mitralign lock; the resulting tension brings the leaflets closer together, reducing the annular circumference. Another direct annuloplasty device is the Valtech Cardioband, in which access is transseptal; this has been used exclusively to treat functional MR.
Since patients treated by the MitraClip may suffer recurrence of their original grade of MR in the medium to long term, the idea of associating annuloplasty is an attractive one, since it more closely reproduces surgical repair, and appears to be the most promising approach22 while we await the development of a prosthetic mitral valve that can be implanted percutaneously in the native valve.
ConclusionsOur initial experience with the MitraClip device confirmed the safety of the procedure in a group of patients with heart failure and grade 4+ functional MR who were not considered suitable for surgery, three of whom would have been excluded from the EVEREST II trial and four of whom had been considered for heart transplantation.
The short follow-up does not allow a proper analysis of the device's efficacy, but the absence of reintervention (surgical or otherwise), improvements in functional class, left ventricular volumes and six-minute walk test, and consistent falls in proBNP levels, all suggest that it is at least reasonable. However, MitraClip implantation does not appear to alter the natural history of the disease in patients under consideration for heart transplantation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cacela D, Fiarresga A, Branco L, et al. Terapêutica percutânea da insuficiência mitral: experiência inicial com o dispositivo MitraClip. Rev Port Cardiol. 2015;34:515–524.