Cardiac surgery for structural heart disease (often involving the left atrium) and radiofrequency catheter ablation of atrial fibrillation have led to an increased incidence of regular atrial tachycardias, often presenting as atypical flutters. This type of flutter is particularly common after pulmonary vein isolation, especially after extensive atrial ablation including linear lesions and/or defragmentation.
The authors describe the case of a 51-year-old man, with no relevant medical history, referred for a cardiology consultation in 2009 for paroxysmal atrial fibrillation. After failure of antiarrhythmic therapy, he underwent catheter ablation, with criteria of acute success. Three years later he again suffered palpitations and atypical atrial flutter was documented. The electrophysiology study confirmed the diagnosis of atypical left flutter and reappearance of electrical activity in the right inferior pulmonary vein. This vein was again ablated successfully and there has been no arrhythmia recurrence to date.
In an era of frequent catheter ablation it is essential to understand the mechanism of this arrhythmia and to recognize such atypical flutters.
A existência de cardiopatia estrutural, a história de cirurgia cardíaca prévia (frequentemente envolvendo a aurícula esquerda) e a ablação percutânea da fibrilhação auricular conduziram a um aumento da incidência de taquicardias auriculares regulares, frequentemente apresentando-se como flutters atípicos. Este tipo de flutter é particularmente frequente após isolamento das veias pulmonares, especialmente após extensa ablação auricular, incluindo lesões e/ou desfragmentação lineares.
Os autores descrevem o caso de um homem, 51 anos de idade, sem antecedentes patológicos conhecidos, encaminhado para a consulta de cardiologia, em 2009, por fibrilhação auricular paroxística. Após insucesso da terapêutica antiarrítmica, foi referenciado para ablação percutânea, com critérios de sucesso agudo. Três anos depois, reiniciou queixas de palpitações e foi documentado flutter auricular atípico. O estudo eletrofisiológico confirmou o diagnóstico de flutter atípico esquerdo e reaparecimento de atividade elétrica na veia pulmonar inferior direita. Fez re-ablação da veia recanalizada com sucesso, sem qualquer recorrência arrítmica até ao momento.
Na era da ablação percutânea, é essencial perceber o mecanismo da arritmia e reconhecer a existência destes flutters atípicos.
The authors describe the case of a 51-year-old man, referred for a cardiology consultation in 2009 for paroxysmal atrial fibrillation (AF), the first episode of which was documented in December 2008, lasting 18 hours and terminating spontaneously; he had no other relevant medical history. Further investigation by transthoracic echocardiography revealed no structural heart disease, normal cardiac chamber dimensions, and preserved biventricular systolic function with no evidence of diastolic or valve dysfunction; laboratory tests, including renal, thyroid and liver function, showed no significant alterations. Exercise testing (Bruce protocol lasting 8 min 30 s, rate-limited, asymptomatic) revealed no significant electrocardiographic alterations or evidence of cardiac rhythm disturbances during exertion or recovery. In 2010 he reported several episodes of palpitations (European Heart Rhythm Association [EHRA] classification III), and AF was documented on Holter ambulatory monitoring. Initially these episodes occurred around once a month. Therapeutic options were discussed with the patient and it was decided to begin antiarrhythmic therapy with flecainide and bisoprolol; since his CHA2DS2VASc score was 0, antithrombotic therapy was not prescribed.
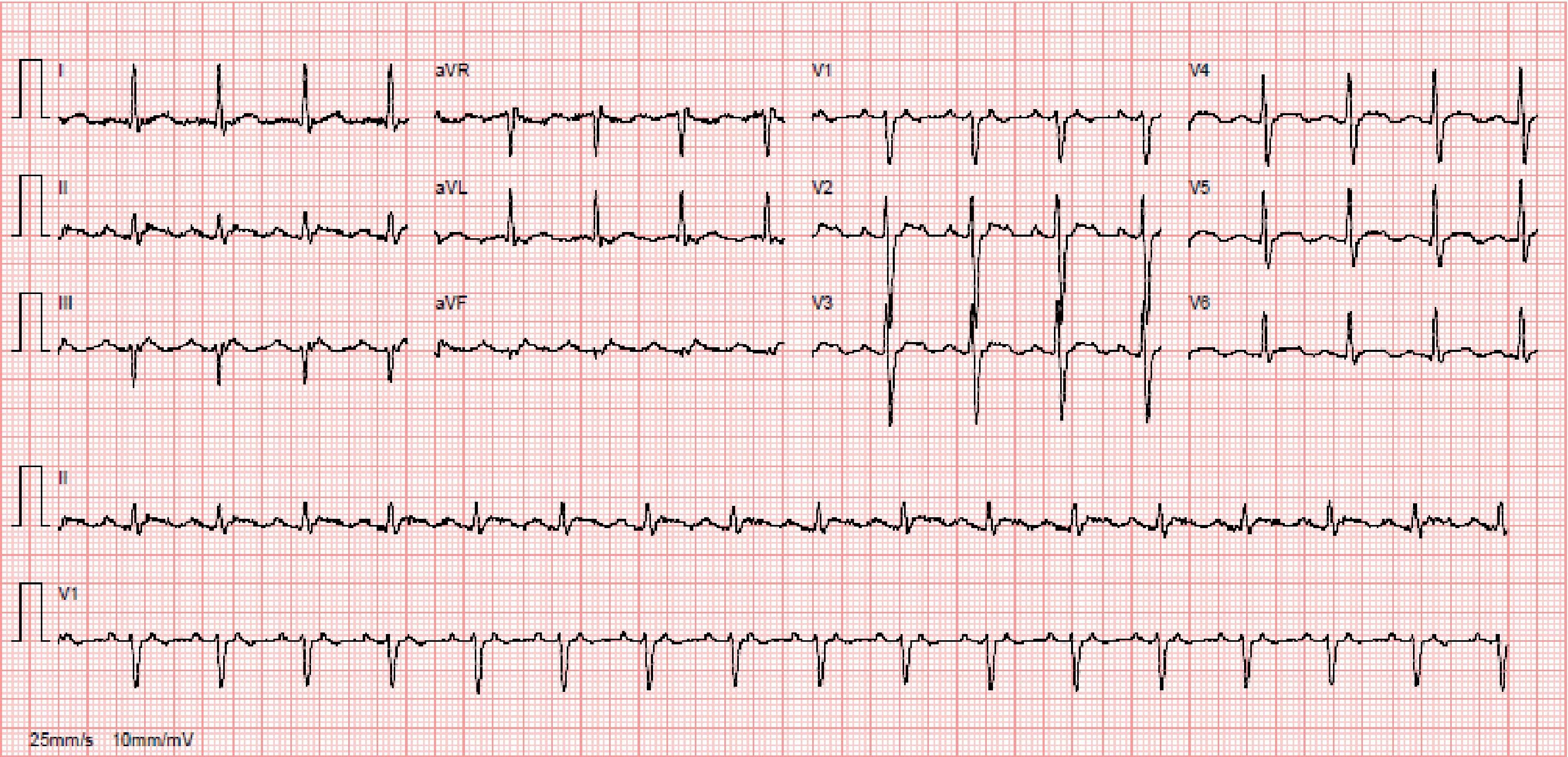
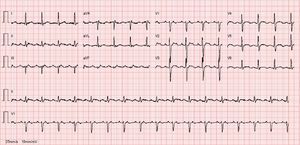
Despite antiarrhythmic therapy, the patient had several symptomatic recurrences of AF and was therefore referred for catheter ablation. In 2011 he underwent radiofrequency pulmonary vein isolation, with criteria of acute success. In 2014 he again suffered palpitations and went to the emergency department, where atypical atrial flutter (AFl) with rapid ventricular response was documented (Figure 1). During this episode he was prescribed an anticoagulant, heart rate control was begun with bisoprolol, and electrical cardioversion was scheduled for three weeks later. This was performed successfully, anticoagulation was maintained for four weeks and antiarrhythmic therapy was again prescribed with propafenone and bisoprolol. However, symptomatic AFl recurred several times and it was therefore decided to perform electrophysiology study (EPS) and AFl ablation.
In March 2015 another EPS was performed immediately preceded by computed tomography angiography (CTA) of the left atrium and pulmonary veins, even though their anatomy was known from the previous procedure, to quantify left atrial volumes, which also excluded pulmonary vein stenosis. The CTA images were used for electroanatomical mapping of the left atrium during the EPS, although the images were not integrated.
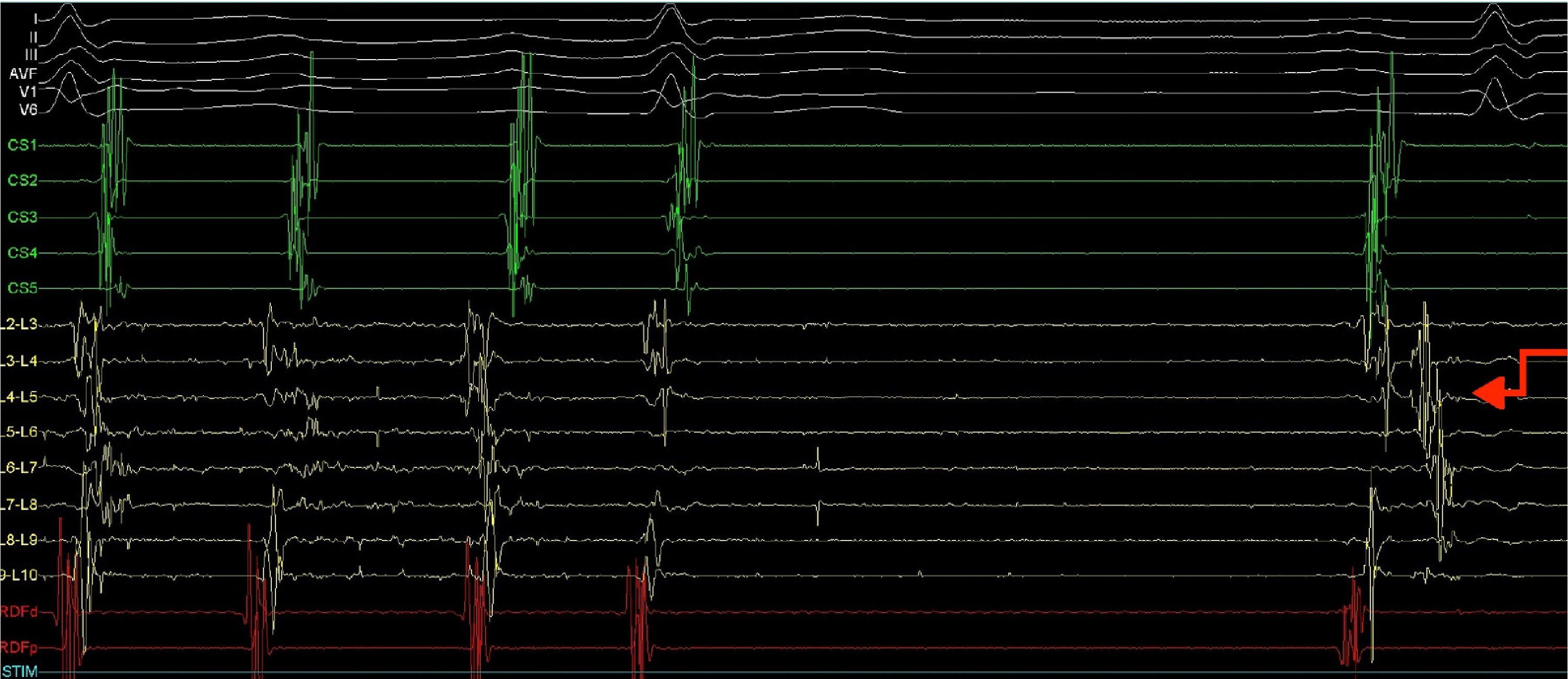
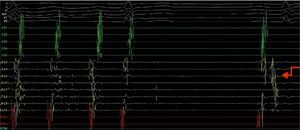
A quadripolar catheter was introduced into the right atrium or His position and a decapolar catheter was introduced into the coronary sinus, both by femoral vein catheterization. Based on the previous pulmonary vein isolation and the algorithms for locating the circuit according to the morphology of flutter (F) waves on the surface electrocardiogram (ECG), a double transseptal approach was immediately performed, introducing a decapolar circular catheter (Lasso, Biosense) and an irrigated-tip ablation catheter with pressure sensor (SmartTouch), which were advanced to the left atrium. As the Lasso catheter was being manipulated near the inferior pulmonary vein, to map the anatomy of the left atrium, the AFl ceased due to mechanical block (Figure 2).
Electrophysiology study demonstrating conversion of left atrial flutter to sinus rhythm by simply manipulating the Lasso catheter in the left atrium next to or inside the left inferior pulmonary vein, and showing a pulmonary vein signal in the first sinus beat. CS1-CS5: decapolar catheter in the coronary sinus; (L2-L3) to (L9-L10) decapolar circular Lasso catheter; RDFd-RDFp: ablation catheter. Arrow: pulmonary vein signals.
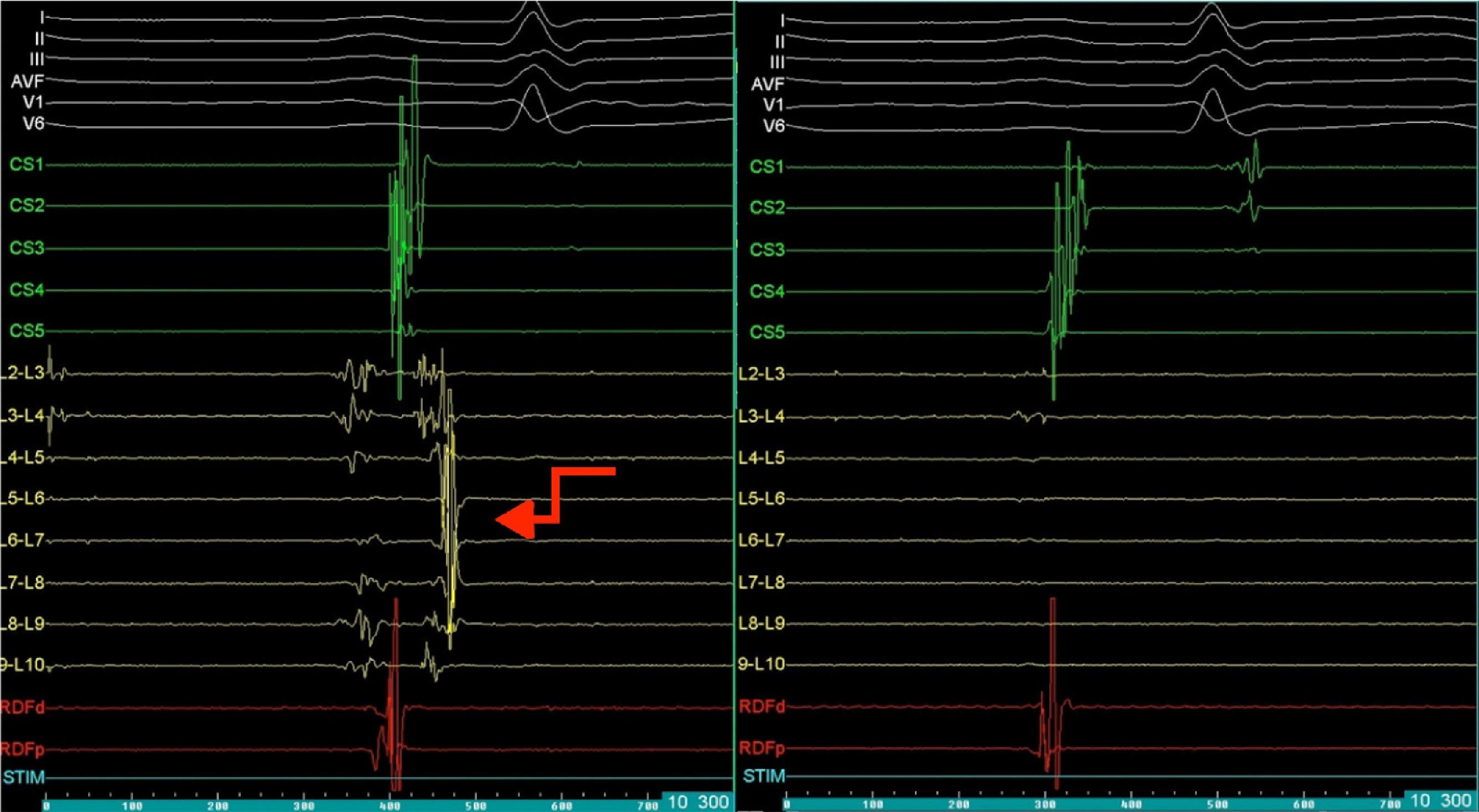
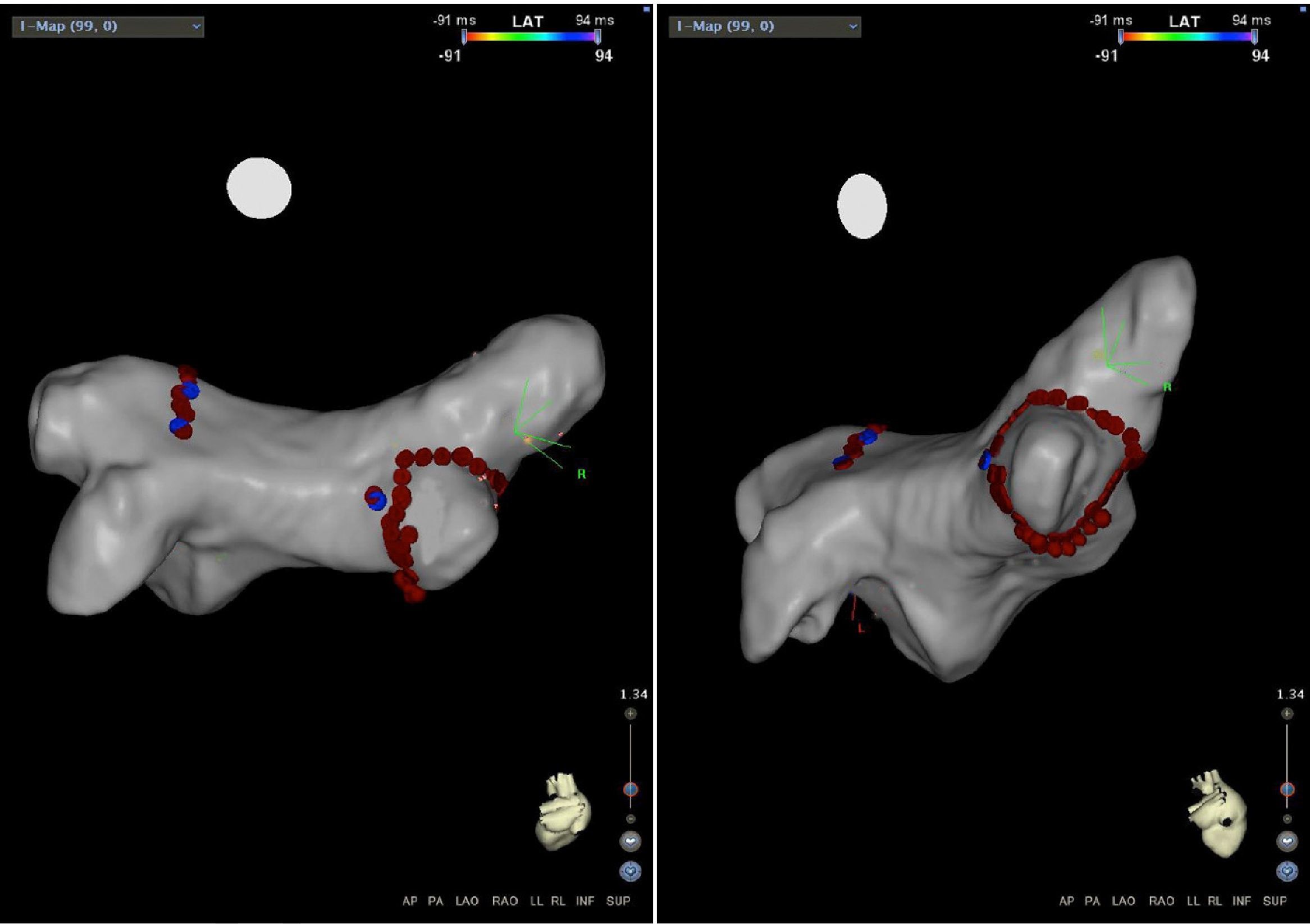
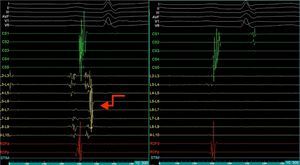
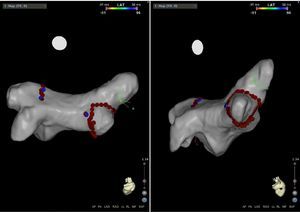
Mapping of the left atrium only showed electrical activity in the right inferior pulmonary vein (Figure 3). Programmed electrical stimulation of the atrium was performed with three base cycles and three coupled extrastimuli until the refractory period without AFl or AF being induced. Since AFl was not inducible and could therefore not be mapped in detail, the right inferior pulmonary vein was isolated, with criteria of acute success and no periprocedural complications (Figure 4).
Electrophysiology study demonstrating electrical activity in the right inferior pulmonary vein (left) and no electrical activity following re-isolation of the vein (right). CS1-CS5: decapolar catheter in the coronary sinus; (L2-L3) to (L9-L10) decapolar circular Lasso catheter; RDFd-RDFp: ablation catheter. Arrow: pulmonary vein signals.
At six-month follow-up the patient reported clear symptomatic improvement, with only sporadic palpitations. Repeat 24-hour electrocardiographic monitoring revealed sinus rhythm and nine supraventricular extrasystoles.
DiscussionAF is a major cardiovascular challenge in modern society. Its prevalence is estimated at 1.5-2% of the general population and it is associated with increased risk for stroke and congestive heart failure and thus high mortality.1,2
According to the European Society of Cardiology guidelines, catheter ablation of symptomatic paroxysmal AF is recommended in patients who have symptomatic recurrence of AF on antiarrhythmic drug therapy (class I recommendation, level of evidence A).1 Pulmonary vein isolation is considered the reference treatment in catheter ablation.1–3
There have been significant technical advances in catheter ablation of AF in recent years, and it has become one of the most widely used treatments for this arrhythmia. However, success rates vary considerably, which may be due to differences in population characteristics, techniques, and assessment criteria.4
Ablation is more effective in maintaining sinus rhythm than antiarrhythmic drugs in AF, but long-term recurrence rates are significant. Previous studies report success rates of 85% at one year and 52% at five years for radiofrequency ablation. Early recurrence appears to be the main negative predictor, but age, left atrial dimensions, structural heart disease, and type and duration of AF are also factors in recurrence.1,2,4
Wei et al. studied 267 patients treated by pulmonary vein isolation and found that in a mean follow-up of 300 days, 44 patients (16.5%) showed recurrence, of which 21 had AFl, 18 AF, and five atrial tachycardia. Multivariate analysis revealed that AF type, AF duration, left atrial diameter and left pulmonary vein diameter were risk factors for recurrence (hazard ratios of 3.416, 2.148, 4.619 and 2.811, respectively, p<0.05).4
Cryoablation has gained increasing acceptance for pulmonary vein isolation. Although first-generation cryoablation balloons showed only moderate long-term efficacy and an acceptable safety profile (the main adverse effects being phrenic nerve paralysis), second-generation devices (28 mm balloon incorporating a modified refrigerant injection system providing homogeneous cooling of the complete distal balloon hemisphere) demonstrated 80% success in a one-year follow-up (81% in paroxysmal AF and 77% in short-term persistent AF). The incidence of esophageal injury ranges between 12% and 19%, and the incidence of phrenic nerve palsy varies considerably between centers (3.5-19.5%).5
AFl can occur after catheter AF ablation. The term ‘flutter’ was first used in 1887 by John McWilliam, who described the visual phenomena resulting from atrial stimulation as “a rapid flutter”.6 A macroreentrant mechanism was finally proven by detailed mapping in the electrophysiology laboratory.7
AFl is less common than AF, but its prevalence also increases with age. The incidence of AFl in those younger than 50 years is 5/100000 and 587/100000 in those >80 years old.7
AFl can coexist with or precede AF, and the relation between the two is complex. Some authors have shown that patients with coexistent AF and AFl benefit from catheter ablation of AF, possibly associated with AFl ablation instead of AFl ablation only.7
In 1970 Puech and Grolleau proposed a classification of AFl according to 12-lead electrocardiographic morphology. The most frequent form of flutter, termed ‘common’, was characterized by predominantly negative biphasic F waves in the inferior leads with a sawtooth pattern, and predominantly positive F waves in V1; AFL was termed ‘atypical’ if the electrocardiographic morphology was different from the common type.7
AFl is currently defined as a regular atrial tachycardia with a rate of ≥240 bpm lacking an isoelectric baseline between deflections. It is termed typical (cavotricuspid isthmus-dependent) if the inferior pivot point is the area bounded anteriorly by the inferior part of the tricuspid orifice, and posteriorly by the inferior part of the vena cava orifice.1,7
Cardiac surgery for structural heart disease (often involving the left atrium) and radiofrequency catheter ablation of atrial fibrillation have led to an increased incidence of regular atrial tachycardias, often presenting as atypical flutters. This type of flutter is particularly common after pulmonary vein isolation, especially after extensive atrial ablation including linear lesions and/or defragmentation. Gaps in prior ablation lines may also play a role in reentrant circuits. The most common mechanisms are perimitral, roof-dependent and septal circuits; recently, biatrial circuits have also been reported.7,8
The most useful method for determining the origin (left or right) of AFl from the surface ECG is assessment of F-wave morphology in V1. F waves in this lead with a broad positive base are strongly predictive of a left origin, while an initially isoelectric or inverted component followed by a positive deflection is compatible with right AFl. Deeply inverted F waves in V1 are highly suggestive of a right origin. Finally, biphasic or isoelectric F waves in V1 do not help in identifying the chamber of origin.9
In the case presented, the patient's background and the electrocardiographic characteristics of the AFl (F waves with broad positive base in V1) suggested from the outset that it was atypical left AFl. Since mitral AFl is one of the most common types seen after AF ablation, it was considered a possible diagnosis in view of the electrocardiographic features, but this could not be confirmed by EPS since a sustainable AFl could not be induced and could therefore not be mapped in detail. EPS only revealed electrical activity in the right inferior pulmonary vein, which was successfully re-isolated.
An example of an atypical left AFl is thus perimitral AFl, caused by a macroreentrant circuit around the mitral annulus and areas of low voltage or scarring in the left atrium. Gerstenfeld et al. analyzed the electrocardiographic characteristics of mitral flutters. The polarity in each lead was determined by examining the F-wave during the 80 ms before the tallest peak positive deflection in V1. Counterclockwise mitral flutter was positive in the inferior and precordial leads and had a negative component in leads I and aVL. Clockwise mitral flutter demonstrated a significant negative F-wave in the inferior leads and positive F-wave in leads I and aVL.7 An electrophysiologic diagnosis of perimitral flutter is obtained by pacing techniques using a single multipolar catheter in the coronary sinus when the post-pacing interval is <30 ms in the proximal as well as the distal coronary sinus.7
The European and American guidelines recommend catheter ablation of symptomatic non-cavotricuspid isthmus-dependent AFl refractory to antiarrhythmic drug therapy (class IIa recommendation).8
Successful ablation of atypical AFl depends on identifying the critical part of the reentrant circuit that can be interrupted by radiofrequency application. Mapping systems enable three-dimensional reconstruction of the sequence of atrial activation during tachycardia and localization of areas of scarring or conduction block. In patients who have undergone previous cardiac surgery, the surgical report is useful in identifying the possible location of reentrant circuits near surgical incisions in the atrium.7,8
Perimitral AFl is particularly difficult to treat, since bidirectional mitral isthmus block has a limited effect on recurrence and can be a challenge to achieve in the acute phase, requiring potentially dangerous epicardial ablation in the coronary sinus, and significant late conduction recovery is seen in up to 60% of cases despite complete acute block. Ablation of a modified anterior mitral line has recently been shown to be safe and effective compared to the lateral line, improving the success rate of mitral isthmus block.8,10
With this case, the authors highlight the importance of catheter ablation of AFl nowadays and the forms in which arrhythmias may recur during follow-up. In our patient there was recurrence three years after pulmonary vein isolation in the form of atypical left AFl of unknown location. The case also demonstrates that in AFl following pulmonary vein isolation when ablation lines are not created, the circuits responsible are usually related to recovery of electrical activity in one or more pulmonary veins, with the microreentrant circuit located close to one of them, and with centrifugal activation of both atria. Effective treatment consists simply of re-isolation of the pulmonary veins involved.
To conclude, the case presented is a good example of the above approach, since our patient is symptom-free, in sinus rhythm and not taking antiarrhythmic medication, six months after a simple re-ablation of the right inferior pulmonary vein, the only one to show recovery of conduction, even though the reentrant circuit involved in the genesis of this atypical AFl could not be mapped in detail.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferreira R, Primo J, Adão L, Gonzaga A, Gonçalves H, Santos R, et al. Flutter auricular atípico tardio após ablação de fibrilhação auricular. Rev Port Cardiol. 2016;35:539.