Oxidative stress appears to play a critical role in the pathogenesis of preeclampsia. Evidence suggests that adequate intake of antioxidants can modulate this condition. The objective of this study was to assess the intake of antioxidant nutrients and coefficients of variation in pregnant women with preeclampsia.
MethodsIn a cross-sectional study in the public health network of the city of Maceió, Brazil, a dietary survey was performed consisting of 24-hour food recalls, with subsequent adjustment of nutrients using the estimated average requirement as the cutoff point, and a questionnaire on frequency of consumption of antioxidants.
ResultsWe studied 90 pregnant women with preeclampsia (PWP) and 90 pregnant women without preeclampsia (PWoP) with mean ages of 25.8±6.7 years and 24.1±6.2 years (p=0.519), respectively. A low mean intake of antioxidants (vitamin A, selenium, zinc and copper) was observed in both PWP and PWoP, although intakes of vitamin A (p=0.045) and selenium (p=0.008) were higher in PWoP. In addition, we observed high coefficients of variation in nutrient intakes in both groups, which were higher for vitamin C (p<0.001), vitamin A (p=0.006) and copper (p=0.005) in PWP.
ConclusionsConsumption of antioxidant nutrients by pregnant women with preeclampsia is inadequate, with considerable daily variations in intake, which points to a need for nutrition education strategies aimed at improving intakes, because diet is without doubt a key factor in the modulation of oxidative stress caused by preeclampsia.
O estresse oxidativo é uma provável via crítica na patogênese da pré-eclâmpsia. Evidências têm sugerido que o consumo adequado de antioxidantes é capaz de modular essa condição. Assim, o objetivo da presente pesquisa foi avaliar a ingestão e o coeficiente de variabilidade de nutrientes antioxidantes por gestantes com pré-eclâmpsia (GCP).
MétodosEstudo transversal realizado na rede pública de saúde do município de Maceió através de inquérito dietético, com aplicação de: recordatórios alimentares de 24 horas, com posteriores ajustes dos nutrientes pelo método da EAR como ponto de corte, e questionário de frequência de consumo de antioxidantes.
ResultadosForam estudadas 90 GCP e 90 gestantes sem pré-eclâmpsia (GSP), com médias de idade de 25,8±6,7 anos e 24,1±6,2 anos (p=0,519), respectivamente. Foram observadas baixas médias de consumo de antioxidantes (vitamina A, selênio, zinco e cobre) para GCP e GSP, apesar do maior consumo de vitamina A (p=0,045) e selênio (p=0,008) pelas GSP. Adicionalmente, foram observados elevados coeficientes de variabilidade de consumo para ambos os grupos (GCP versus GSP, respectivamente); no entanto, maiores para as GCP de vitamina C (p<0,001), vitamina A (p=0,006) e cobre (p=0,005).
ConclusõesO consumo de nutrientes antioxidantes pelas GCP é inadequado, somado às elevadas variações diárias no seu consumo, resultado que revela a necessidade do desenvolvimento de estratégias de educação nutricional, no sentido de adequar a ingestão, pois a dieta é, sem dúvida, um fator essencial na modulação do estresse oxidativo causado pela condição de pré-eclâmpsia.
Preeclampsia is the leading cause of maternal mortality in Brazil, affecting 3-17% of pregnancies. Furthermore, it is one of the main causes of admission to intensive care units,1–3 and is associated with increased risk for maternal and fetal adverse events.4
Preeclampsia is a metabolic disorder characterized by elevated blood pressure that develops after the 20th week of pregnancy associated with proteinuria of ≥0.3 g in a 24-hour urine collection. It leads to endothelial damage, platelet aggregation, activation of the coagulation system, increased vascular resistance and oxidative stress.1,5–7
Oxidative stress is defined as oxidation of macromolecules or other cell components resulting from increased levels of reactive oxygen and nitrogen species (RONS), and/or weakened antioxidant defenses, leading to cell damage. It plays a critical role in the pathogenesis of preeclampsia, as demonstrated by the presence of biomarkers of oxidative stress.8
Cells have various mechanisms to protect against oxidative stress and effective antioxidant defenses will prevent cell damage. Antioxidants are classified as enzymatic and nonenzymatic; the latter are also known as dietary antioxidants and include vitamins C, E and A, the trace elements zinc, selenium and copper, and phytochemicals such as flavonoids.9
It is not easy to determine individuals’ diet, since eating habits are inseparable from symbolic aspects of social life and have a variety of meanings at all levels from the cultural environment to individual experiences, and it is therefore harder to perform an objective analysis of food habits by standard research methods. Nonetheless, dietary surveys are valuable tools for relating diet to health and disease.10
Considering that antioxidants reduce the levels of RONS associated with oxidative stress, which is a characteristic of preeclampsia, the objective of this study is to assess the intake of antioxidant nutrients and coefficients of variation (CVs) in a population of pregnant women with preeclampsia.
MethodsThis was a case-control study carried out in 2014 in the public health network of the city of Maceió, Brazil, of pregnant women with preeclampsia referred to Professor Alberto Antunes University Hospital (HUPAA), the reference center for high-risk pregnancies in the state of Alagoas, and normotensive pregnant women attending prenatal consultations in primary health centers (PHCs) in the city. Those who were not resident in the city, bedridden, with neurological disorders, in serious condition or not being treated at HUPAA or in PHCs in Maceió, were excluded.
Sample size was calculated using Epi Info, version 7.0, based on a prevalence of preeclampsia of 17%,11 for a 90% confidence level, 80% power and 1:1 ratio of exposed to non-exposed subjects. The estimated sample size was 178, composed of 89 pregnant women with preeclampsia (PWP) and 89 pregnant women without preeclampsia (PWoP).
A standardized questionnaire, previously tested by our group, was applied, including socioeconomic characteristics (income, education and self-reported skin color), personal and family history of preeclampsia, anthropometric data (pre-pregnancy weight, current weight and height), and eating habits (24-hour food recall and a semi-quantitative food frequency questionnaire [SQFFQ]).
The diagnosis of preeclampsia was confirmed by consultation of medical records and defined as the presence of systemic hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg) and proteinuria of ≥0.3 g in a 24-hour urine collection after the 20th week of pregnancy.5
Anthropometric assessment consisted of height and weight measurement using a digital scale (Filizola®) and a portable stadiometer. Body mass index (BMI) was calculated using the cutoff points established by Atalah et al.12 Pre-pregnancy weight and BMI were also assessed, as was weight gain during pregnancy adjusted for gestational age according to the guidelines of the US Institute of Medicine.13
Eating habits were assessed through two 24-hour food recalls, the first at the time of application of the questionnaire and the second by telephone two weeks later. The food recalls were analyzed using Avanutri® 4.0 nutritional assessment and prescription software and nutrients and calories were subsequently adjusted using the estimated average requirement (EAR) as the cutoff point.14 Assessment of the adequacy of intake was based on the guidelines for preeclampsia in the case of nutrients specifically mentioned for the condition and on dietary reference intakes (DRIs) for other nutrients. Intakes were considered adequate when the mean of the two food recalls was between the EAR corresponding to the DRI and the tolerable upper intake level. The CV of each nutrient in the subjects’ diet was also calculated.15
The SQFFQ used by Rohenkohl et al.16 was adapted and applied to all study participants in order to assess intake of food sources of five antioxidants: vitamins A, C and E, beta-carotene, and selenium. Daily and weekly frequency of intake of sources of these antioxidants was assessed. Weekly intakes were rated on a scale of 0 to 4, with 0 corresponding to no consumption, 1 to less than once a week, 2 to once or twice a week, 3 to two or three times a week and 4 to more than five times a week; these were further grouped as frequent (>2 times a week) or infrequent intake (≤2 times a week). The SQFFQ was designed to assess intakes of dietary sources of reasonable quantities of these nutrients that are frequently consumed by the general population, as well as foods defined as important sources of these antioxidants in food composition tables. The questionnaire was applied once only to each study participant. Weekly intakes were assessed on the basis of food sources rich in antioxidants, as follows: (1) sources of vitamin A: butter, carrots, fish oil, cheese, liver, eggs and milk; (2) sources of vitamin C: bell peppers, guava, kiwi fruit, oranges, lemons, cabbage, asparagus and acerola; (3) sources of vitamin E: butter, egg yolk, corn and sunflower oil, mayonnaise, hazelnuts, almonds and wheatgerm; (4) sources of beta-carotene: broccoli, apricots, papayas, mangoes, nutmeg, spinach, tomatoes, sweet potatoes, carrots, and dark green and orange vegetables; and (5) sources of selenium: brazil nuts, sunflower seeds, liver, eggs, chicken, cheese, fish and shellfish.
The statistical analysis was performed with Epi Info version 7.0. The chi-square test and the t test were used to compare frequencies and means, respectively, of the food habits of PWP and PWoP.
The study was approved by the ethics and research committee of the Federal University of Alagoas (UFAL), protocol number 341.953.
ResultsThe study population consisted of 90 PWP and 90 PWoP, with mean ages of 25.8±6.7 years and 24.1±6.2 years (p=0.519), respectively, and mean gestational ages at the time of assessment of 30.1±8.3 weeks and 23.2±9.1 weeks, respectively.
In terms of the demographic and socioeconomic characteristics of PWP and PWoP, respectively, 17.8% vs. 27.8% (p=0.096) were aged ≤19 years and 8.9% vs. 10.0% (0.592) were aged ≥35 years; 43.3% vs. 45.5% (p=0.433) had <4 years of schooling; and 30.0% vs. 24.4% (p=0.407) had low income (less than the minimum salary).
Concerning the nutritional status of PWP and PWoP, respectively, 14.4% vs. 17.7% (p=0.678) were underweight and 40.1% vs. 13.3% (p<0.001) were obese according to BMI during pregnancy, and 45.5% vs. 50.0% (p=0.705) presented inadequate weight gain during pregnancy compared to 34.5% vs. 16.7% (p=0.013) with excessive weight gain during pregnancy.
With regard to the presence of diseases in PWP and PWoP, respectively, 35.5% vs. 0.0% had chronic disease (p<0.001), of whom 29/90 (32.2%) had chronic hypertension, 12/90 (13.3%) had diabetes and 1/90 (1.1%) had sickle cell anemia, while in terms of obstetric history, this was the first pregnancy in 40.0% vs. 42.2% (p=0.762), 38.9% vs. 1.1% (p<0.001) had a history of preeclampsia, and 25.6% vs. 22.2% (p=0.600) reported terminations or miscarriages in previous pregnancies. Only one non-hypertensive woman had had a previous multiple birth pregnancy.
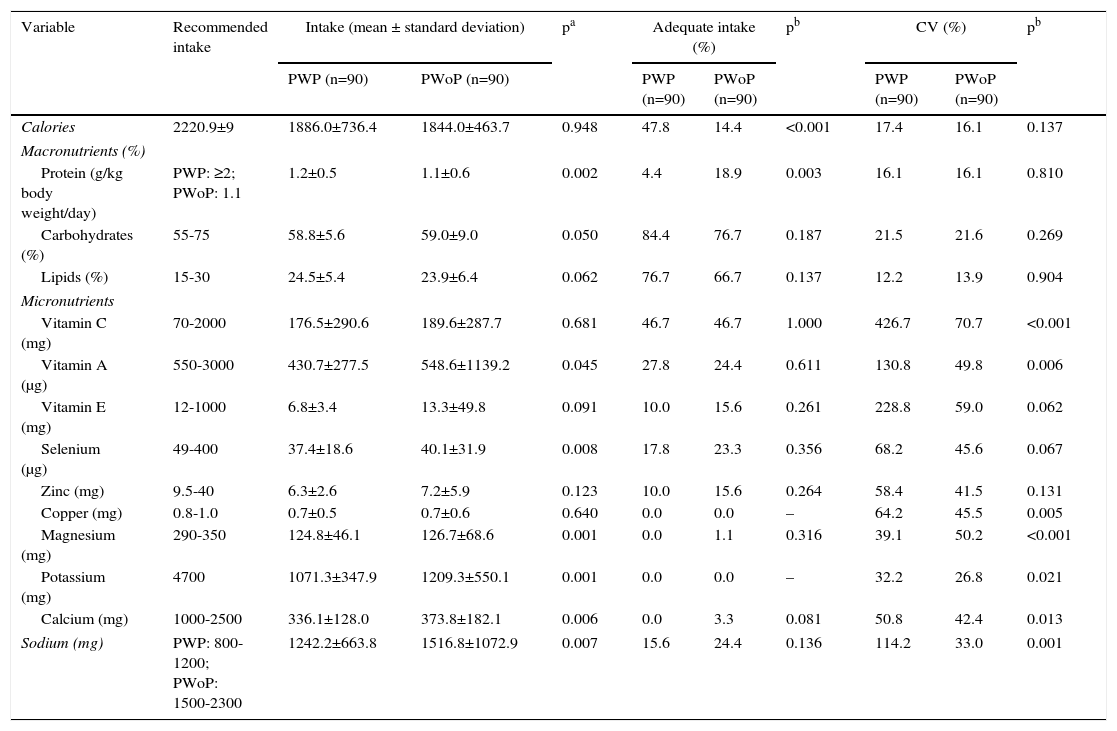
The mean nutrient intakes in the study population are shown in Table 1. Intakes below recommended levels were observed in both groups for calories, vitamin A, selenium, zinc, copper, magnesium, potassium and calcium, although intakes of vitamin A (p=0.045), selenium (p=0.008), magnesium (p=0.001) and calcium (p=0.006) were higher in PWoP. Mean intakes of vitamin C were adequate in both groups and protein, vitamin E and sodium intakes were adequate in PWoP. By contrast, mean sodium intake was higher than recommended in PWP.
Intake of nutrients and coefficients of variation in pregnant women with and without preeclampsia in Maceió, Alagoas, 2014.
| Variable | Recommended intake | Intake (mean ± standard deviation) | pa | Adequate intake (%) | pb | CV (%) | pb | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PWP (n=90) | PWoP (n=90) | PWP (n=90) | PWoP (n=90) | PWP (n=90) | PWoP (n=90) | |||||
| Calories | 2220.9±9 | 1886.0±736.4 | 1844.0±463.7 | 0.948 | 47.8 | 14.4 | <0.001 | 17.4 | 16.1 | 0.137 |
| Macronutrients (%) | ||||||||||
| Protein (g/kg body weight/day) | PWP: ≥2; PWoP: 1.1 | 1.2±0.5 | 1.1±0.6 | 0.002 | 4.4 | 18.9 | 0.003 | 16.1 | 16.1 | 0.810 |
| Carbohydrates (%) | 55-75 | 58.8±5.6 | 59.0±9.0 | 0.050 | 84.4 | 76.7 | 0.187 | 21.5 | 21.6 | 0.269 |
| Lipids (%) | 15-30 | 24.5±5.4 | 23.9±6.4 | 0.062 | 76.7 | 66.7 | 0.137 | 12.2 | 13.9 | 0.904 |
| Micronutrients | ||||||||||
| Vitamin C (mg) | 70-2000 | 176.5±290.6 | 189.6±287.7 | 0.681 | 46.7 | 46.7 | 1.000 | 426.7 | 70.7 | <0.001 |
| Vitamin A (μg) | 550-3000 | 430.7±277.5 | 548.6±1139.2 | 0.045 | 27.8 | 24.4 | 0.611 | 130.8 | 49.8 | 0.006 |
| Vitamin E (mg) | 12-1000 | 6.8±3.4 | 13.3±49.8 | 0.091 | 10.0 | 15.6 | 0.261 | 228.8 | 59.0 | 0.062 |
| Selenium (μg) | 49-400 | 37.4±18.6 | 40.1±31.9 | 0.008 | 17.8 | 23.3 | 0.356 | 68.2 | 45.6 | 0.067 |
| Zinc (mg) | 9.5-40 | 6.3±2.6 | 7.2±5.9 | 0.123 | 10.0 | 15.6 | 0.264 | 58.4 | 41.5 | 0.131 |
| Copper (mg) | 0.8-1.0 | 0.7±0.5 | 0.7±0.6 | 0.640 | 0.0 | 0.0 | – | 64.2 | 45.5 | 0.005 |
| Magnesium (mg) | 290-350 | 124.8±46.1 | 126.7±68.6 | 0.001 | 0.0 | 1.1 | 0.316 | 39.1 | 50.2 | <0.001 |
| Potassium (mg) | 4700 | 1071.3±347.9 | 1209.3±550.1 | 0.001 | 0.0 | 0.0 | – | 32.2 | 26.8 | 0.021 |
| Calcium (mg) | 1000-2500 | 336.1±128.0 | 373.8±182.1 | 0.006 | 0.0 | 3.3 | 0.081 | 50.8 | 42.4 | 0.013 |
| Sodium (mg) | PWP: 800-1200; PWoP: 1500-2300 | 1242.2±663.8 | 1516.8±1072.9 | 0.007 | 15.6 | 24.4 | 0.136 | 114.2 | 33.0 | 0.001 |
CV: coefficient of variation; PWP: pregnant women with preeclampsia; PWoP: pregnant women without preeclampsia.
Table 1 also reveals that the nutrients for which both groups had the highest percentages of adequate intake were carbohydrates (84.4% vs. 76.7%, p=0.187) and lipids (76.7% vs. 66.7%, p=0.137), while the lowest percentages were seen for potassium (0.0% vs. 0.0%), copper (0.0% vs. 0.0%), magnesium (0.0% vs. 1.1%, p=0.316) and calcium (0.0% vs. 3.3%, p=0.081). PWP presented lower percentages of adequate intake than PWoP for protein (4.4% vs. 18.9%, p=0.003) and higher percentages for calories (47.8% vs. 14.4%, p<0.001), although the percentages with adequate intakes were low in both groups.
The CVs revealed high variation in nutrient intakes in both groups, although higher for vitamin C (426.7% vs. 70.7%, p<0.001), vitamin A (130.85% vs. 49.8%, p=0.006), copper (64.2% vs. 45.5%, p=0.005), calcium (50.8% vs. 42.4%, p=0.013) and sodium (114.2% vs. 33.0%, p=0.001) in PWP; there was lower variation in magnesium intake in PWP (39.1% vs. 50.2%, p=0.001).
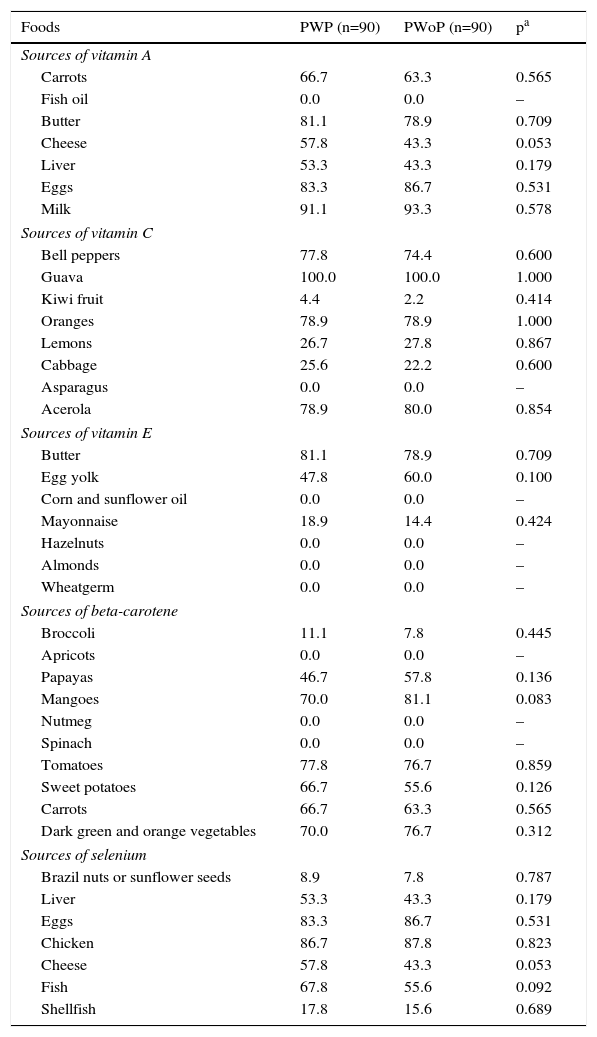
Finally, Table 2 presents the percentages of pregnant women who frequently consumed sources of antioxidants. No significant differences were found between PWP and PWoP. The most frequently consumed sources of antioxidants were as follows: sources of vitamin A: milk (91.1% vs. 93.3%, p=0.578), eggs (83.3% vs. 86.7%, p=0.531) and butter (81.1% vs. 78.9%, p=0.709); sources of vitamin C: guava (100.0% vs. 100.0%, p=1.000), acerola (78.9% vs. 80.0%, p=0.854) and oranges (78.9% vs. 78.9%, p=1.000); sources of vitamin E: butter (81.1% vs. 78.9%, p=0.709), egg yolk (47.8% vs. 60.0%, p=0.100) and mayonnaise (18.9% vs. 14.4%, p=0.424); sources of beta-carotene: tomatoes (77.8% vs. 76.7, p=0.859), mangoes (70.0% vs. 81.1%, p=0.083) and dark green and orange vegetables (70.0% vs. 76.7%, p=0.312); and sources of selenium: chicken (86.7% vs. 87.8%, p=0.823). eggs (83.3% vs. 86.7%, p=0.531) and fish (67.8% vs. 55.6%, p=0.092).
Percentages of pregnant women with and without preeclampsia who frequently consumed sources of antioxidants in Maceió, Alagoas, 2014.
| Foods | PWP (n=90) | PWoP (n=90) | pa |
|---|---|---|---|
| Sources of vitamin A | |||
| Carrots | 66.7 | 63.3 | 0.565 |
| Fish oil | 0.0 | 0.0 | – |
| Butter | 81.1 | 78.9 | 0.709 |
| Cheese | 57.8 | 43.3 | 0.053 |
| Liver | 53.3 | 43.3 | 0.179 |
| Eggs | 83.3 | 86.7 | 0.531 |
| Milk | 91.1 | 93.3 | 0.578 |
| Sources of vitamin C | |||
| Bell peppers | 77.8 | 74.4 | 0.600 |
| Guava | 100.0 | 100.0 | 1.000 |
| Kiwi fruit | 4.4 | 2.2 | 0.414 |
| Oranges | 78.9 | 78.9 | 1.000 |
| Lemons | 26.7 | 27.8 | 0.867 |
| Cabbage | 25.6 | 22.2 | 0.600 |
| Asparagus | 0.0 | 0.0 | – |
| Acerola | 78.9 | 80.0 | 0.854 |
| Sources of vitamin E | |||
| Butter | 81.1 | 78.9 | 0.709 |
| Egg yolk | 47.8 | 60.0 | 0.100 |
| Corn and sunflower oil | 0.0 | 0.0 | – |
| Mayonnaise | 18.9 | 14.4 | 0.424 |
| Hazelnuts | 0.0 | 0.0 | – |
| Almonds | 0.0 | 0.0 | – |
| Wheatgerm | 0.0 | 0.0 | – |
| Sources of beta-carotene | |||
| Broccoli | 11.1 | 7.8 | 0.445 |
| Apricots | 0.0 | 0.0 | – |
| Papayas | 46.7 | 57.8 | 0.136 |
| Mangoes | 70.0 | 81.1 | 0.083 |
| Nutmeg | 0.0 | 0.0 | – |
| Spinach | 0.0 | 0.0 | – |
| Tomatoes | 77.8 | 76.7 | 0.859 |
| Sweet potatoes | 66.7 | 55.6 | 0.126 |
| Carrots | 66.7 | 63.3 | 0.565 |
| Dark green and orange vegetables | 70.0 | 76.7 | 0.312 |
| Sources of selenium | |||
| Brazil nuts or sunflower seeds | 8.9 | 7.8 | 0.787 |
| Liver | 53.3 | 43.3 | 0.179 |
| Eggs | 83.3 | 86.7 | 0.531 |
| Chicken | 86.7 | 87.8 | 0.823 |
| Cheese | 57.8 | 43.3 | 0.053 |
| Fish | 67.8 | 55.6 | 0.092 |
| Shellfish | 17.8 | 15.6 | 0.689 |
PWP: pregnant women with preeclampsia; PWoP: pregnant women without preeclampsia.
Nutritional intervention is extremely important in preeclampsia, since it can improve prognosis, especially by ensuring adequate intake of antioxidants, due to the apparent relationship between the disease and increased oxidative stress.8
As can be seen, the study population presented unfavorable socioeconomic conditions, to be expected for populations using the public health network.17 Studies have shown that poorer populations usually have less access to nutritionally adequate and safe foods, since they are less able to afford them.18 This could explain the limited diversity of consumption of the main food groups in this study, as demonstrated by low mean intakes and high percentages of inadequate intakes for most of the nutrients studied. This was independent of the existence of preeclampsia, although for some nutrients intakes were even lower in PWP (Table 1).
Mean intakes of antioxidant vitamins in PWP were below recommended levels, with high CVs and low percentages of adequate intake of vitamins A and E. This is of concern, given both the low levels and high variation in intakes of these nutrients.14 In addition, the most frequently consumed sources of vitamin E in both groups were butter, egg yolk and mayonnaise, and the most frequently consumed sources of vitamin A were milk, eggs and butter, but butter, milk and mayonnaise contain high levels of saturated or trans fats, which are potentially atherogenic.19 On the other hand, the study revealed frequent consumption of mangoes, tomatoes and dark green and orange vegetables in both groups, and these are natural sources of beta-carotene, which besides having antioxidant activity20 is also a precursor of vitamin A in humans.21
By contrast, mean vitamin C intake was within recommended limits, despite the low percentages with adequate intake and high CVs, which were considerably higher in PWP than in PWoP (426.7% vs. 70.7%, p<0.001). The fact that mean intake of this vitamin was adequate can be explained by the fact that recommended vitamin C levels are generally easily achieved when fruit with high levels of the vitamin are part of the regular diet. Qualitative and quantitative analysis of the study population's diet shows that certain vitamin C-rich fruit, such as guava, acerola and oranges, are commonly consumed in this region. Even so, the CV for consumption of this vitamin was high, suggesting that there was considerable variation in intakes in this population, since the higher the CV the greater the range, and hence greater uncertainty as to whether individual intakes were adequate.14
There is considerable interest in antioxidant supplementation in preeclampsia, particularly the potential of vitamins C and E to control and even prevent the disease, but studies have produced conflicting results. Nevertheless, these nutrients should be consumed as part of a healthy diet.22 A recent systematic review suggested that a diet rich in fruit and vegetables may have beneficial effects in preeclampsia,23 since these foods are natural sources of antioxidants, highlighting the importance of a balanced diet in this context.
There were also high rates of inadequacy and high daily variations in intakes of selenium, zinc and copper in the present study, and mean selenium intake was significantly lower in PWP (p=0.008). The main sources of these minerals consumed by the study population were chicken, eggs and fish. Their antioxidant effects are due to the fact that they are key components of antioxidant enzymes such as glutathione peroxidase (GPx), which depends on selenium, and the Cu/Zn form of superoxide dismutase (SOD), which depends on copper and zinc.9 In a case-control study assessing levels of zinc, copper and selenium and GPx and SOD in umbilical cord blood samples from preeclamptic and normotensive mothers, decreased levels of these trace elements and antioxidant enzymes were observed in the preeclamptic population,24 highlighting the relationship between these minerals and the proper functioning of these antioxidant enzymes and the need for adequate intakes to prevent complications of the disease.
Besides the antioxidants described above, other nutrients analyzed in this study are essential for the control of preeclampsia, including magnesium, potassium and calcium.22 The women in this study, both PWP and PWoP, presented low mean intakes of these elements, especially in PWP, none of whom had adequate intakes, and in whom higher CVs were seen in intakes of calcium (50.8% vs. 42.4%, p=0.013) and potassium (33.3% vs. 26.8%, p=0.021).
In an overview of the literature, Xu et al.25 found low dietary intakes of magnesium in women with preeclampsia, as in the present study. Magnesium supplementation has been recommended for the prevention and treatment of preeclampsia and eclampsia, with some success. Studies on magnesium levels in pregnant women with and without preeclampsia have revealed significant differences in magnesium homeostasis, suggesting that the mineral may play an important role in the prevention and treatment of the disease. A possible mechanism is its anti-inflammatory effect, since studies have shown that magnesium reduces the production of inflammatory cytokines, which is a feature of the pathophysiology of preeclampsia and eclampsia. Other studies indicate that it reduces blood pressure by altering nitric oxide synthesis, and it has been suggested that low magnesium intake alters the prostacyclin/thromboxane ratio, which also affects preeclampsia. However, although some studies show a beneficial effect of magnesium supplementation in pregnancy, further research is needed before this can be generally recommended.26,27
The role of calcium in the etiology of preeclampsia has been demonstrated in epidemiological studies showing an association between low calcium intake and high prevalence of the disease. Supplementation with 2 g/day calcium has been associated with lower risk for preeclampsia and lower blood pressure levels in hypertensive pregnant women. Together with other ions (sodium, magnesium and potassium), calcium helps maintain normal blood pressure. A physician considering calcium supplementation for either prevention or treatment of preeclampsia should take into account the cost/benefit ratio, the bioavailability of the mineral, and the patient's dietary intake.28,29
A noteworthy finding of our study was the low proportion of PWP (15.6%) with sodium intakes within the recommended limits for the disease, as well as the high CVs in PWP compared to PWoP (114.2% vs. 33.0%, p=0.001), given that control of sodium intake is a basic element of dietary recommendations in preeclampsia.5,22 Although sodium is essential for regulation of extracellular fluid, in preeclampsia it reduces glomerular filtration rate, increases sensitivity to angiotensin, has vasoconstrictive effects and reduces atrial natriuretic peptide levels.5,22,23
Mean protein intake in the study population was higher in PWP (1.2±0.5 vs. 1.1±0.6 g/kg body weight/day, p=0.002), but a lower percentage of PWP had adequate protein intake (4.4% vs. 18.9%, p=0.002), which may reflect the use of a cutoff for the high-protein diet recommended in preeclampsia (≥2 g/kg body weight/day) aimed at correcting hypoproteinemia and promoting normal fetal development.22 The aim of a high-protein diet, with an increased proportion of high biological value protein, is to enhance endogenous production of albumin, the protein responsible for maintaining fluid balance between the intravascular and interstitial fluid compartments. It is thought that normalizing albumin levels is more effective in reducing edema and blood pressure in preeclampsia than sodium restriction. A high-protein diet is also recommended for other essential functions in pregnancy besides fluid and electrolyte balance, including lipid transport and tissue synthesis.22,23
Among the limitations of the study were the fact that only two (the recommended minimum) 24-hour food recalls were applied, and its cross-sectional nature, which means that no causal relationships can be established between the presence of preeclampsia and the nutritional factors under analysis.
ConclusionConsumption of antioxidant nutrients by pregnant women with preeclampsia is inadequate, with considerable daily variations in intake, which points to a need for nutrition education strategies aimed at improving intakes, because diet is without doubt a key factor in the modulation of oxidative stress caused by preeclampsia.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Cabral Menezes de Oliveira A, Albuquerque Santos A, Rodrigues Bezerra A, Machado Tavares MC, Rocha de Barros AM, Costa Ferreira R. Ingestão e coeficiente de variabilidade de nutrientes antioxidantes por gestantes com pré-eclâmpsia. Rev Port Cardiol. 2016;35:469–476.