In this report, we present the case of an adult male with severe mitral regurgitation due to an atrioventricular septal defect. Anatomical assessment by two- and three-dimensional transesophageal echocardiography was essential for detailed morphological characterization and surgical planning. The different features of a ‘cleft’ in an atrioventricular septal defect compared to an anterior leaflet cleft in an otherwise normal mitral valve are here discussed.
Os autores apresentam o caso de um doente adulto com regurgitação mitral severa no contexto de um defeito do septo auriculoventricular. A avaliação conjunta por ETE 2 D e 3 D foi fundamental para a caracterização anátomo-funcional da válvula e orientação do procedimento cirúrgico. As particularidades anatómicas de uma «fenda» em contexto de defeito do septo auriculoventricular e de uma fenda numa válvula mitral são aqui discutidas.
Atrioventricular septal defect (AVSD) covers a range of congenital cardiac malformations that have in common a single atrioventricular (AV) junction. This results in significant morphological alterations, partial AVSD being characterized by a common AV junction but two separate AV valves due to the fusion of the superior and inferior leaflets (bridging leaflets), which gives the left AV valve a trifoliate configuration. This is different from mitral valve clefts not associated with endocardial cushion defects.1
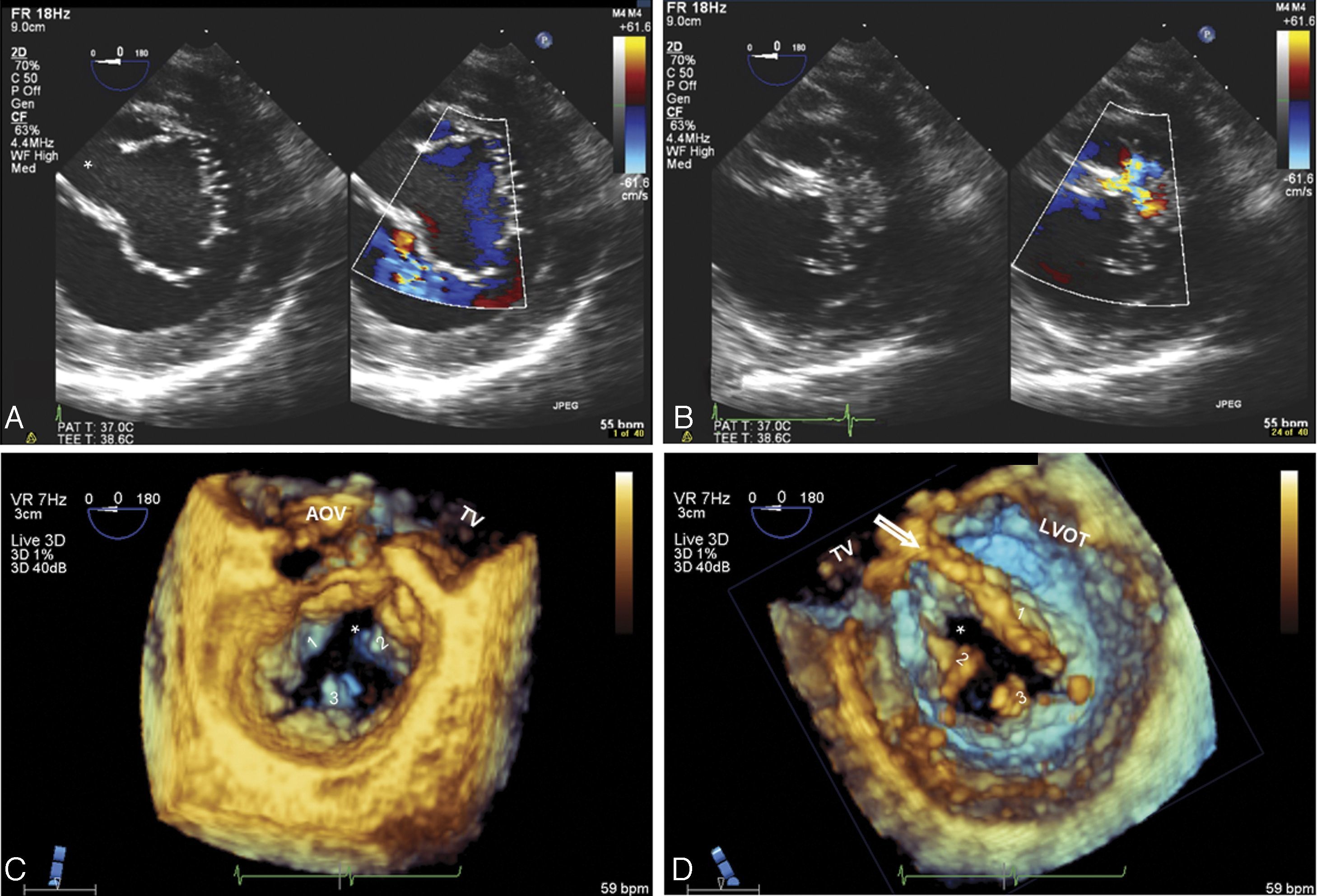
Case reportA 52-year-old man was referred for valve surgery due to severe mitral regurgitation secondary to a mitral cleft and moderate aortic regurgitation through a bicuspid aortic valve. Two-dimensional transesophageal echocardiography (2D TEE) showed a single AV junction with a trifoliate left valve: one mural, one anterosuperior and one posteroinferior, with a ‘cleft’ oriented towards the interventricular septum in the zone of apposition (Figure 1A and B). The papillary muscles were displaced laterally, next to the mural leaflet commissures with bridging leaflets. Three-dimensional (3D) TEE provided detailed anatomical information (Figure 1C and D). No evidence of interatrial or interventricular communication, usually characteristic of AVSD, was observed.
(A and B) Two-dimensional transesophageal echocardiography, transgastric view, showing trifoliate configuration of the left AV valve and the ‘cleft’ (*) in diastole (A); mitral regurgitation from the ‘cleft’ in systole (B); (C and D) real-time three-dimensional zoom, cropped images: in diastole, atrial view (C) and ventricular view (D), the morphology of the three leaflets of the left AV valve can be seen in detail, as well as the septal orientation (towards the right ventricle) of the ‘cleft’. Abnormal chordal attachments linking the edges of the ‘cleft’ to the interventricular septum can be clearly seen in ventricular view (arrow). Other features of a left atrioventricular valve can be seen – elliptical annulus and small mural leaflet. 1: anterosuperior leaflet; 2: posteroinferior leaflet; 3: mural leaflet; AOV: aortic valve; LVOT: left ventricular outflow tract; TV: tricuspid valve.
Both valves were reconstructed. The mitral valve was repaired by direct suturing of the ‘cleft’ using separate 6-0 polypropylene sutures, enlarging the posterior leaflet with an autologous pericardial patch, and annuloplasty using a 28 mm semi-rigid Physio ring (Carpentier-Edwards, Edwards Lifesciences). Aortic regurgitation was corrected by plicating the noncoronary leaflet and reducing the annulus by annuloplasty of the aortoventricular junction. Postoperative TEE showed minimal regurgitation and a mean left atrial/left ventricular gradient of 5 mmHg.
DiscussionUnlike the normal heart that has separate left and right AV junctions, the distinguishing feature of AVSD is a common AV junction – there is no AV septum and the AV annuli are in the same anatomical plane,2 and there is no morphologically normal AV valve. In total AVSD there is a single common AV valve consisting of five leaflets, while in partial AVSD the valve is usually trifoliate, arising from the fusion of the superior and inferior leaflets and consisting of the anterosuperior, posteroinferior and mural leaflets, with three commissures designated anterolateral, posterolateral and septal according to their respective positions. This fusion results in a ‘cleft’ that causes varying degrees of valvular regurgitation, although valve stenosis is rare. Chordal abnormalities are also frequently observed, linking the edges of the ‘cleft’ to the interventricular septum.
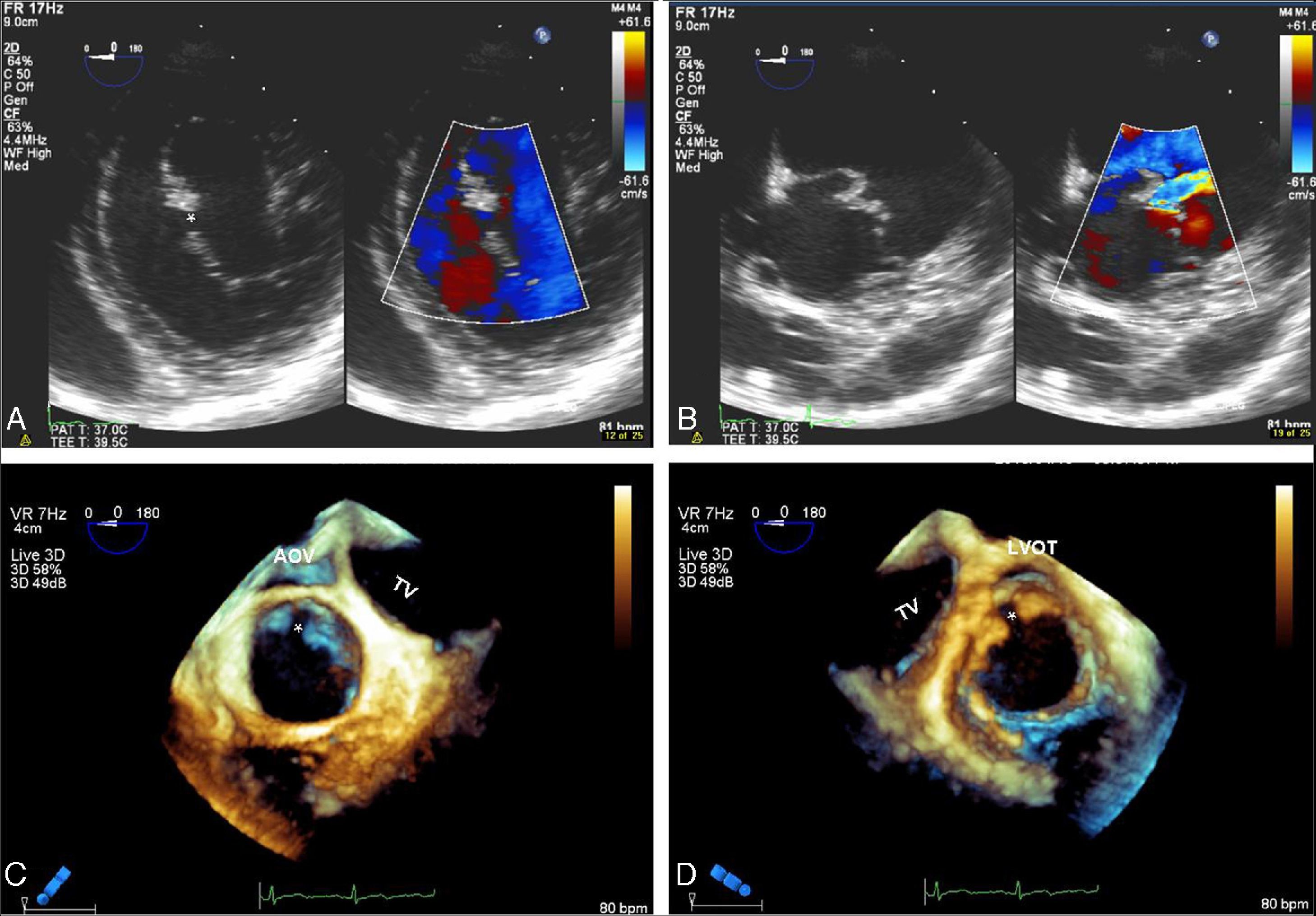
This morphology is completely different from the clefts observed in structurally normal mitral valves, which are more common in the anterior leaflet and the solution of continuity not only does not divide the leaflet into two separate parts but is oriented towards the left ventricular outflow tract (Figure 2).
(A and B) Two-dimensional transesophageal echocardiography, transgastric view, showing mitral valve anterior leaflet cleft (*) in diastole (A) and in systole (B); (C and D) real-time three-dimensional zoom, cropped images: in diastole, anterior leaflet cleft in atrial view (C) and ventricular view (D). Note the typical orientation of the cleft towards the left ventricular outflow tract.
The distinction is important since it implies different surgical approaches. In AVSD ‘clefts’, simply closing the edges by direct suturing or with a patch does not transform the left AV valve into an anatomically normal valve.3 Annular dilatation and a degree of central restriction usually cause residual regurgitation. Resolving this and avoiding valve stenosis generally require the use of corrective techniques other than the usual commissuroplasty or direct closure of the ‘cleft’ and placement of a prosthetic ring. Increasing anterior leaflet mobility through excision of the chordae of the accessory commissure, together with total closure with or without the use of an autologous pericardial patch, and enlarging the posterior leaflet with an autologous pericardial patch normally lead to a good final functional result.
In order to avoid damage to conduction tissue, it should be borne in mind that in AVSD the AV node is posteroinferiorly displaced from the tip of the triangle of Koch.4
In the case presented, although most features of the AVSD were identified by 2D TEE, 3D TEE was essential for detailed characterization of the AV valve, which was important for planning the surgical approach to valve reconstruction and highlights the importance of 3D study to guide surgical repair.5
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Moreno N, Almeida J, Amorim MJ. Defeito do septo auriculoventricular num doente adulto. Há «fendas» e fendas. Rev Port Cardiol. 2016;35:181.e1–181.e4.