Rotors and complex fractionated atrial electrograms (CFAEs) have been suggested as possible therapeutic targets in ablation of atrial fibrillation (AF). The aim of this study was to assess the relationship between rotors and CFAEs in patients with AF.
MethodsWe studied 18 patients with AF (mean age 69±8 years, 33% female) who underwent rotor ablation and pulmonary vein isolation. Endocardial mapping was performed with a basket catheter to identify the presence, number and location of rotors and CFAEs. The FIRM™ (Abbott) and CARTO™ (Biosense) systems were used with overlapping of frames from continuous 30-s recordings. CFAEs were classified as stable if present in >15 frames, moderately stable if present in 10-15 frames and unstable if present in 5-9 frames.
ResultsA total of 44 rotors and 60 CFAEs (39 of them stable) were identified. The mean number of rotors and stable CFAEs per patient was 2.6±1.4 and 2.2±1.5, respectively. In 27 of the 44 identified rotors, CFAEs were found in the same location. Conversely, in 20 of the 39 stable CFAEs identified, a focal rotor was found in the same location. The majority of CFAEs found at the same location as a focal rotor were stable (63% vs. 37%, p=0.001).
ConclusionRotors and CFAEs are frequently found in the same location within the atria, particularly when only stable CFAEs are considered. This relationship may have implications in the selection of substrate targets for ablation.
Os rotores e potenciais complexos fracionados (CFAE) têm sido apontados como possíveis alvos terapêuticos na ablação de fibrilhação auricular (FA). O objetivo deste estudo foi avaliar a relação entre CFAE e rotores em doentes com FA.
MétodosForam estudados 18 doentes com FA (idade média 69±8, 33% mulheres) submetidos a ablação de rotores e isolamento das veias pulmonares. Efetuou-se mapeamento endocárdico com cateter basket para identificação da presença, número e localização de rotores e CFAEs. Foram utilizados os sistemas FIRM (Abbott) e CARTO (Biosense) com sobreposição de frames obtidos a partir de registos contínuos de 30 segundos. Os CFAEs foram classificados em estáveis se presentes em>15 frames, moderadamente estáveis se presentes em 10-15 frames e pouco estáveis se presentes em 5-9 frames.
ResultadosForam identificados 44 rotores e 60 CFAEs, dos quais 39 eram CFAE estáveis. O número médio de rotores e CFAE estáveis por doente foi de 2,6±1,4 e 2,2±1,5, respetivamente. Em 27 dos 44 rotores identificados, encontraram-se CFAEs na mesma localização. Por outro lado, em 20 dos 39 CFAE estáveis identificados, foi encontrado um rotor na mesma localização. A maioria dos CFAEs encontrados na mesma localização dos rotores eram CFAE estáveis (63% versus 37%, p=0.001).
ConclusõesOs rotors e CFAEs são frequentemente encontrados na mesma localização a nível auricular, especialmente se apenas se considerarem os CFAEs estáveis. Esta relação poderá vir a ter implicações na seleção do substrato arritmogénico passível de ablação.
Pulmonary vein isolation (PVI) is the cornerstone therapy for atrial fibrillation (AF) ablation. However, in patients with non-paroxysmal AF and/or significant left atrial dilatation, results are often suboptimal and the best treatment strategy remains uncertain.1
In order to achieve lasting elimination of AF, many centers have suggested additional substrate ablation, particularly additional linear lesions or ablation of complex fractionated atrial electrograms (CFAEs).2,3 This approach has been questioned in a recent trial,4 but the failure of CFAE ablation to improve outcomes may in part be due to the shortcomings of current mapping algorithms, including limited temporal and spatial sampling.
Several studies have also reported the presence of focal rotors near or distant from pulmonary vein isolation sites. There is some evidence that ablation of this substrate could likewise increase AF-free survival.5 Although rotors and CFAEs have been suggested as possible therapeutic targets, their relationship and importance in the maintenance of AF remain to be established. The aim of this study was to assess the relationship between focal rotors and CFAEs in patients with AF, using a new four-dimensional (4D) electroanatomical mapping method to identify stable CFAEs.
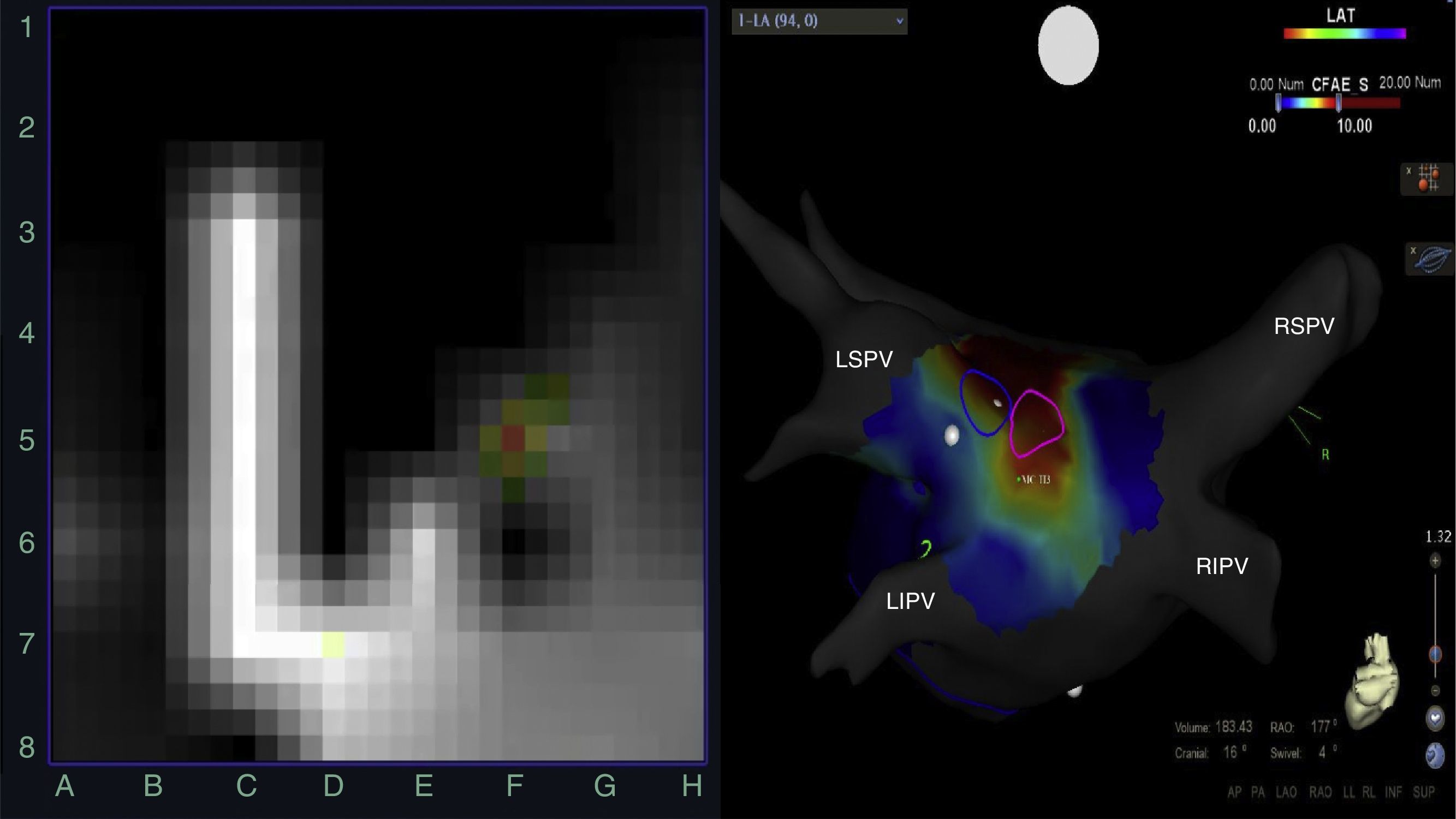
MethodsPopulationEighteen consecutive patients undergoing AF ablation using the FIRM (Abbott, Menlo Park, CA) and CARTO (Biosense Webster, Diamond Bar, CA) electroanatomic mapping systems were included (Figure 1). Patients with severe valvular disease, mechanical prostheses or contraindication for anticoagulation were excluded. The study protocol was approved by the Board of Directors of CHLO. Informed consent to participate in the study was obtained from all patients.
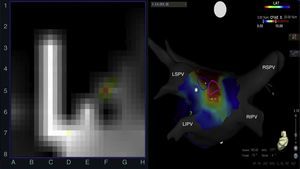
Left: rotor recording with the FIRM system; right: posteroanterior view of the left atrium with CARTO mapping showing rotors (blue and pink circles) over a stable complex fractionated atrial electrogram (red area). CFAE: complex fractionated atrial electrogram; LIPV: left inferior pulmonary vein; LSPV: left superior pulmonary vein; RIPV: right inferior pulmonary vein; RSPV: right superior pulmonary vein.
Before the procedure, patients underwent computed tomographic imaging or transesophageal echocardiography to exclude left atrial thrombus. Conscious sedation was used in all patients. Vascular access was obtained via the femoral veins with two SL1 sheaths (St. Jude Medical, Minneapolis, MN) and a decapolar catheter within the coronary sinus. Transseptal puncture was performed under fluoroscopic guidance and intravenous heparin was infused to maintain activated clotting time >350 s. An anatomical map of the right atrium, left atrium and pulmonary veins was created using the CARTO system (Biosense Webster, Diamond Bar, CA) with a circular mapping catheter (Lasso, Biosense Webster, Diamond Bar, CA). A 64-pole basket catheter (FIRMap® Catheter, Abbott, Menlo Park, CA) was advanced through an 8.5 mm SL1 sheath and deployed first in the right atrium and then in the left atrium.
In patients in sinus rhythm, AF was induced by pacing the atria at a cycle length of 500 ms reduced in 50 ms steps to 300 ms, and then in 10 ms steps.
Computational AF maps were generated using the FIRM system (Abbott, Menlo Park, CA) and electrical rotors, defined as sequential clockwise or counterclockwise activation contours around a center of rotation, were detected and confirmed by visual inspection.
Radiofrequency energy was delivered using a 3.5 mm irrigated-tip catheter (THERMOCOOL SMARTTOUCH SF, Biosense Webster, Diamond Bar, CA) in the areas indicated by FIRM maps to represent the center of rotation activity. PVI was then performed, consisting of wide area circumferential ablation to isolate both veins, and isolation was confirmed using a circular mapping catheter (Lasso, Biosense Webster, Diamond Bar, CA).
AlgorithmThe FIRM and CARTO systems were used with dynamic overlapping of frames obtained from continuous 30-s records. Rotors were defined as previously reported5 and their presence was quantified and localized with the FIRM system in both atria. The CFAE map was obtained offline using automated software from the CARTO system.
CFAEs were calculated by dynamic overlaid mapping using a special algorithm. This algorithm processed ECG recordings divided into 500-ms segments in which each sequence of five segments was considered as a frame (so there was a four-segment overlap between any two consecutive frames). The number of fractionated intervals was calculated for each frame. The dynamic output was the number of fractionated intervals per frame per channel.
The stability of CFAEs was assessed and classified as stable (grade 3) if present in more than 15 frames, moderately stable (grade 2) if present in 10-15 frames and unstable (Grade 1) if present in 5-9 frames.
Statistical analysisContinuous data are represented as medians and interquartile range. Related groups were compared with the Wilcoxon test and summarized with medians and quartiles. Nominal values are expressed as n (%) and compared with chi-square tests or Fisher's exact test for comparisons when the expected cell frequency was <5. A two-sided p value of <0.05 was considered statistically significant throughout.
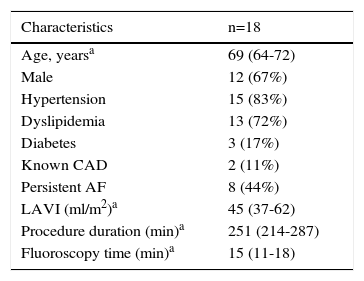
ResultsThe clinical characteristics of the 18 patients included are presented in Table 1. There were no serious complications related to the AF ablation procedure.
Patient characteristics and procedure data.
| Characteristics | n=18 |
|---|---|
| Age, yearsa | 69 (64-72) |
| Male | 12 (67%) |
| Hypertension | 15 (83%) |
| Dyslipidemia | 13 (72%) |
| Diabetes | 3 (17%) |
| Known CAD | 2 (11%) |
| Persistent AF | 8 (44%) |
| LAVI (ml/m2)a | 45 (37-62) |
| Procedure duration (min)a | 251 (214-287) |
| Fluoroscopy time (min)a | 15 (11-18) |
AF: atrial fibrillation; CAD: coronary artery disease; LAVI: left atrial volume index.
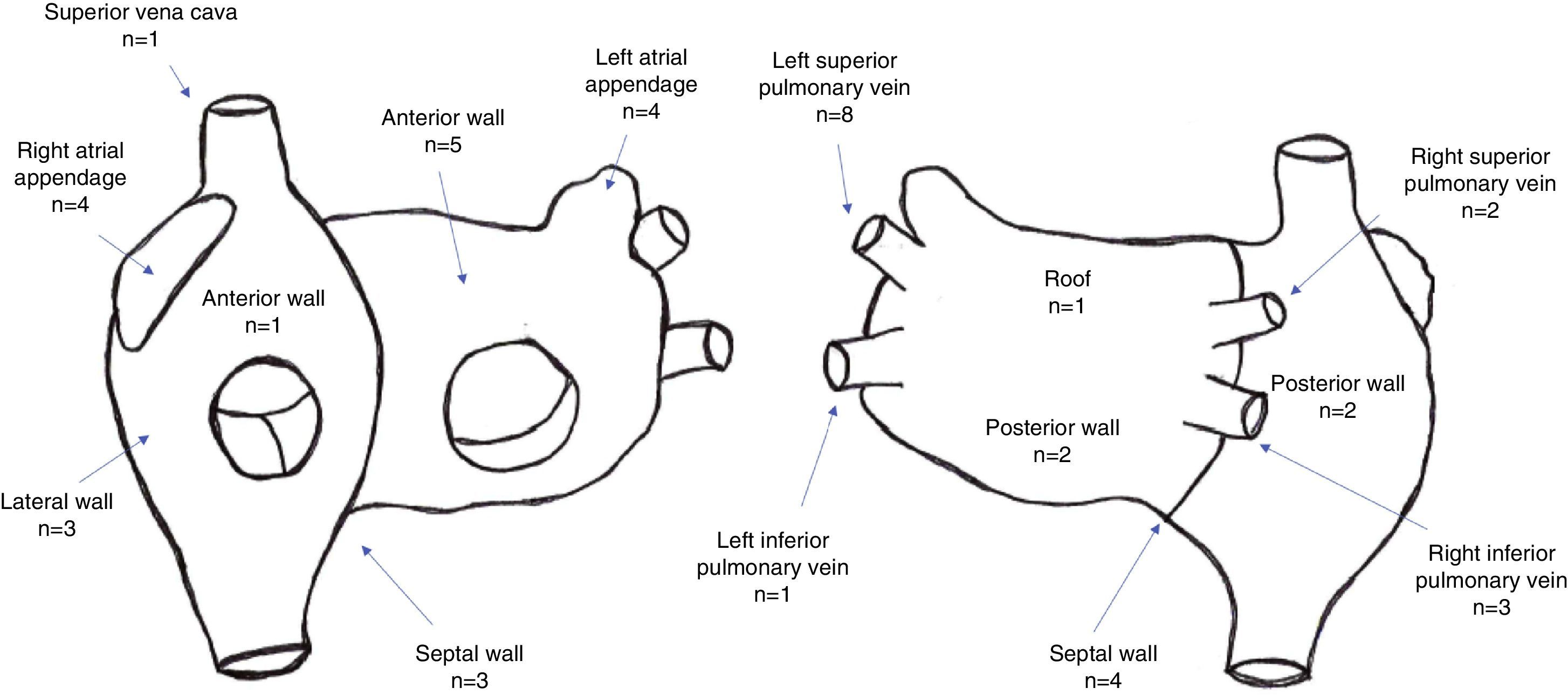
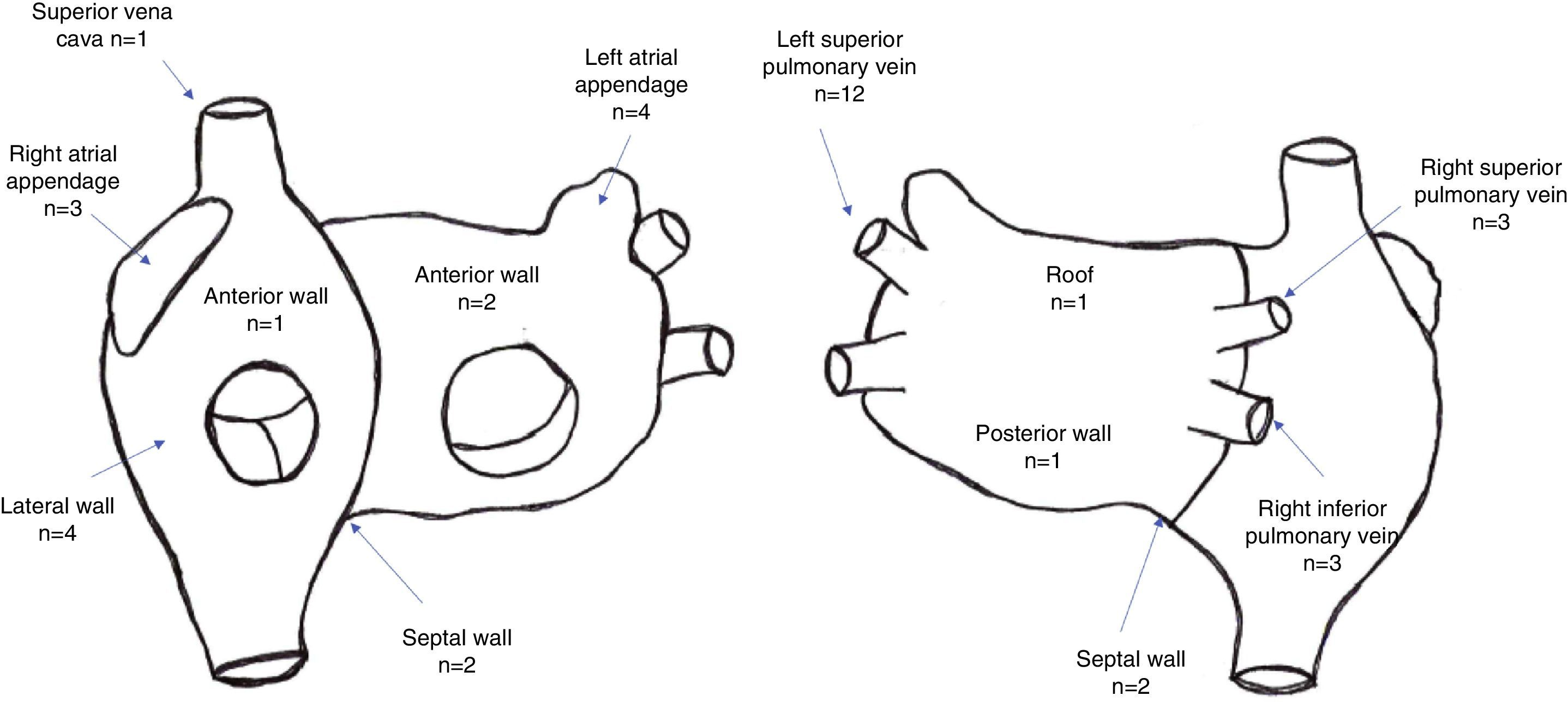
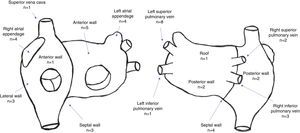
A total of 44 rotors were identified, with a mean of 2.6±1.4 rotors per patient. Thirty rotors were identified in the left atrium and 14 were identified in the right atrium. The location of rotors in both atria is depicted in Figure 2.
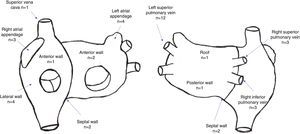
A total of 60 CFAEs (39 CFAE grade 3) were identified, with a mean of 3.6±1.6 CFAEs per patient (mean number of grade 3 CFAEs: 2.2±1.5). Twenty-eight stable CFAEs were identified in the left atrium and 11 stable CFAEs were identified in the right atrium. The location of stable CFAEs in both atria is presented in Figure 3.
Relationship between rotors and complex fractionated atrial electrogramsThe median difference between the number of rotors and the number of CFAEs per patient was -1.0 (-2.0 to 0). The median difference between the number of rotors and the number of stable CFAEs per patient was 0 (-1.0 to 1.3). This difference was significantly smaller for rotors and stable CFAEs compared to rotors and CFAEs overall (p=0.001).
In 20 of 39 stable CFAEs, a rotor was found in the same location (51%), while in 27 of the 44 rotors identified, CFAEs were also present in the same segment (61%). The majority of CFAEs found at the same location as a focal rotor were stable (63% vs. 37%, p=0.001).
DiscussionTwenty years after the first pulmonary vein isolation procedure,6 the underlying mechanisms of AF maintenance remain essentially unknown, and the best ablation strategy remains to be determined. A recent trial4 showed that the two add-on strategies (linear and CFAE ablation) did not increase the success rate of pulmonary vein isolation. Our inability to fully understand the mechanisms of AF persistence and to increase the success rates of PVI may be related to inconsistent analysis of electrical activity in AF. Unlike other regular arrhythmias, AF cannot be mapped using a point-by-point strategy with a few ms of recording. A prolonged three-dimensional (3D) atrial mapping record is probably necessary to understand the underlying mechanisms of AF. To achieve these 4D maps (3D plus time), we adopted the CARTO electroanatomical system and recorded electrical activity with a basket catheter. This catheter is far from ideal due to heterogeneous contact, distortion and reduced steerability, but it does improve the quality of atrial maps compared to traditional catheters (point-by-point) or circular catheters (with limited atrial coverage).
CFAE ablation described by Nademanee et al.2 appeared to be effective in treating paroxysmal and persistent AF, but these results were not reproducible.4 Several factors may account for this failure, including different levels of experience and the lack of specificity and sensitivity of the electrogram recordings and mapping algorithms. Real CFAEs are sometimes difficult to differentiate from unstable electrical activity and far-field artifacts.7 We conceived the possibility of making prolonged records (>30 s) to detect repetitive complex electrograms and enable assessment of CFAE stability.
When studied in this way, only 65% of the CFAEs were shown to be stable according to our criteria. The determinants of CFAE stability are unknown, but we can hypothesize that more stable CFAEs could be a source for AF instead of far-field effects or transient fibrillatory activity.
We used the FIRM system to identify the presence of rotors that were anatomically located in the 3D atrial maps performed with CARTO. Rotors and CFAEs were frequently found in the same location within the atria. The spatial concordance between rotors and CFAEs was greater when only stable CFAEs were considered. The anatomical proximity between the two phenomena may signify that they are different manifestations of the same electrophysiological substrate, pinpointing zones of particular importance in the maintenance of AF. Still, a significant number of CFAEs and rotors are unrelated to each other, suggesting that they may be independent and/or that unstable CFAEs may be the result of far-field effects and that some rotors may be identified as such solely on the basis of subjective assessment of visual observation. Our results appear to contradict previous studies that were unable to show an anatomical relationship between rotors and CFAEs.8–10 This discrepancy could be explained by the novel method we used to identify stable CFAEs, for which prolonged periods of detection may be necessary.
Finally, if confirmed, these findings and mapping methods may improve our understanding of the underlying substrate of AF and assist physicians in the identification of appropriate ablation targets to improve clinical results.
Study limitationsSeveral limitations of this exploratory proof-of-concept study should be acknowledged. The small sample size precludes generalization to the overall population of patients with AF and makes subgroup analysis impracticable. The classification of CFAE stability was largely arbitrary and their relationship with rotors may have been impacted by the inherent subjectivity in the identification of the latter. Further studies will be required to confirm these findings and to assess the clinical impact of ablating substrate targets identified by this new mapping method.
ConclusionRotors and CFAEs are frequently found in the same location within the atria. The spatial concordance between rotors and CFAEs is greater when only stable CFAEs are considered. This relationship may have implications in the selection of appropriate substrate targets for ablation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.