A 76-year-old woman with a medical history notable for factor V gene mutation (with a previous episode of deep venous thrombosis and pulmonary embolism), arthritis and hypertension, on full-dose aspirin (325mg daily) and lisinopril and hydrochlorothiazide (unknown doses), presented in the emergency room complaining of typical 5/10 chest pain, nausea and diaphoresis. She had spent over 5h gardening, which was usual for her. She was given aspirin and nitroglycerin, but the chest pain persisted (intensity 2/10).
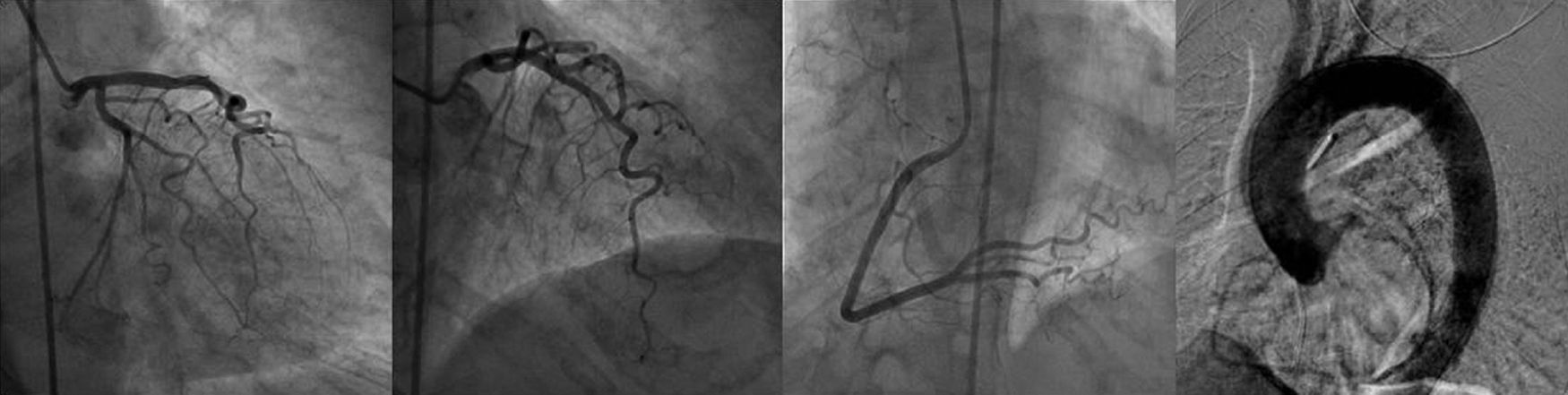
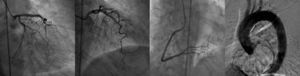
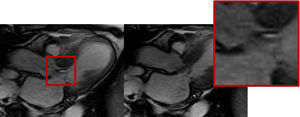
Twelve-lead ECG showed sinus rhythm with ST elevation in leads II, V and VI, and troponin was elevated (2.82ng/ml). She was taken immediately to the cardiac catheterization lab where she was shown to have normal coronary arteries, with a right dominant system, elevated filling pressures and preserved cardiac output, and no evidence of dissection on the ascending aortogram (Figure 1).
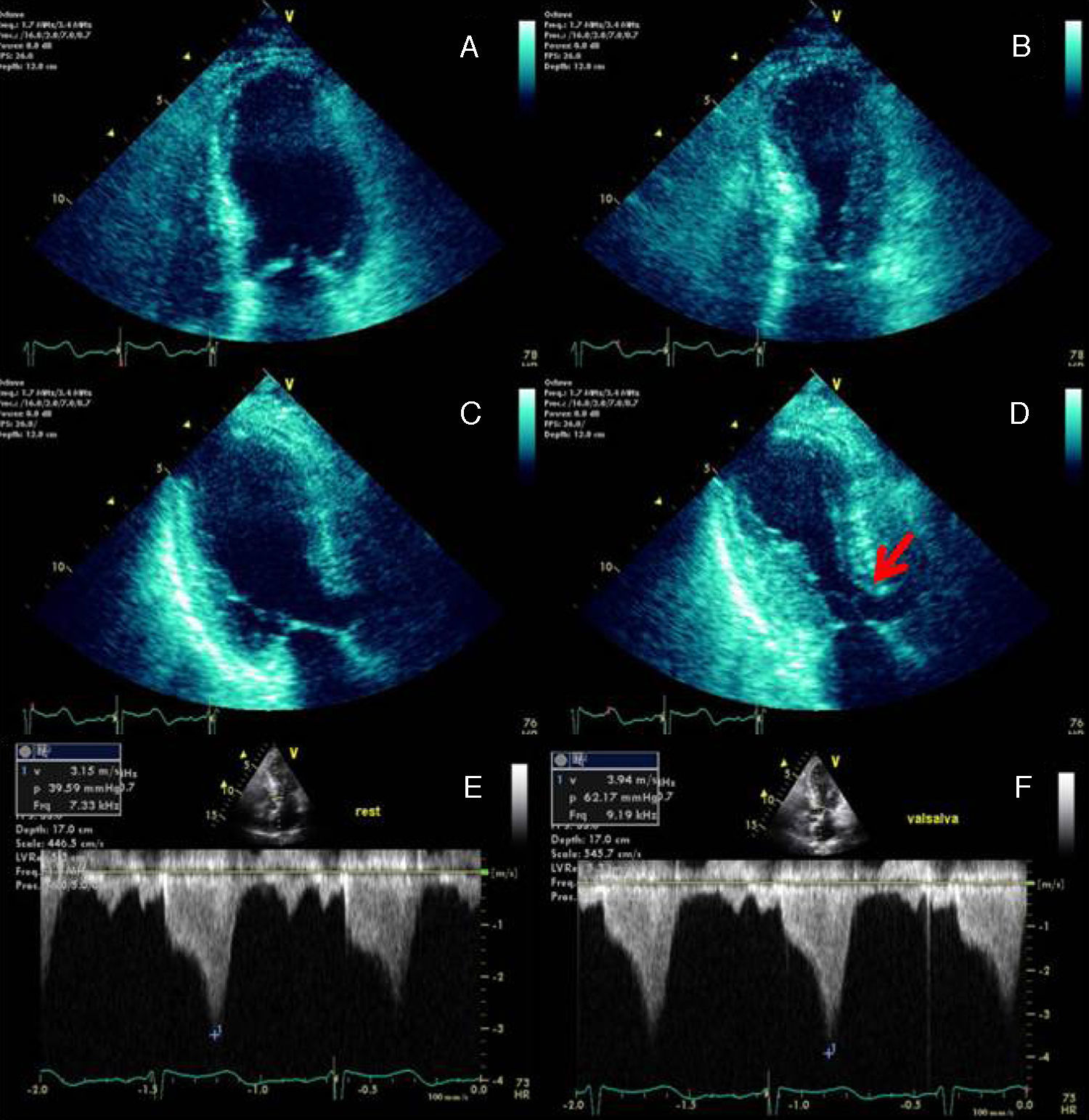
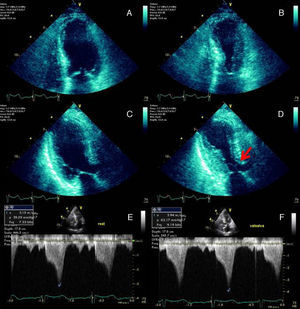
The echocardiogram demonstrated left ventricular (LV) regional wall motion abnormalities (Figure 2), raising the suspicion of myocarditis or Takotsubo cardiomyopathy.
End-diastolic and end-systolic apical four-chamber (A and B) and three-chamber (C and D) echocardiographic views demonstrating the typical apical and mid-ventricular LV wall-motion abnormalities that raised the suspicion of Takotsubo cardiomyopathy. SAM of the mitral valve with LVOT obstruction is signaled by the red arrow (D). Continuous-wave Doppler profile outlining the degree of LVOT obstruction at rest (E) and during the Valsalva maneuver (F).
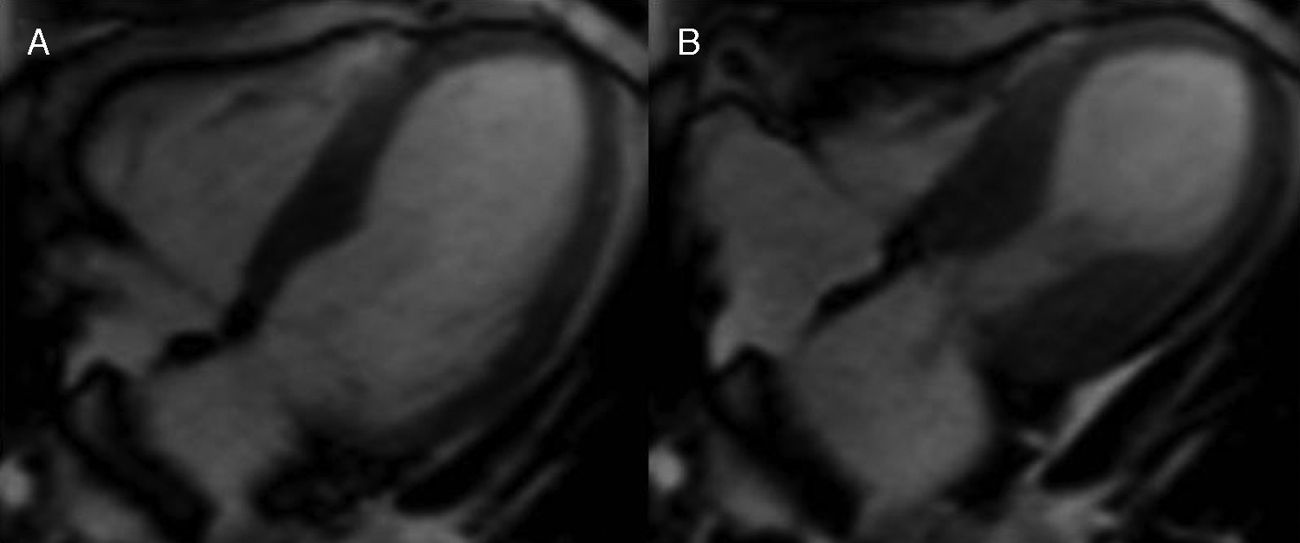
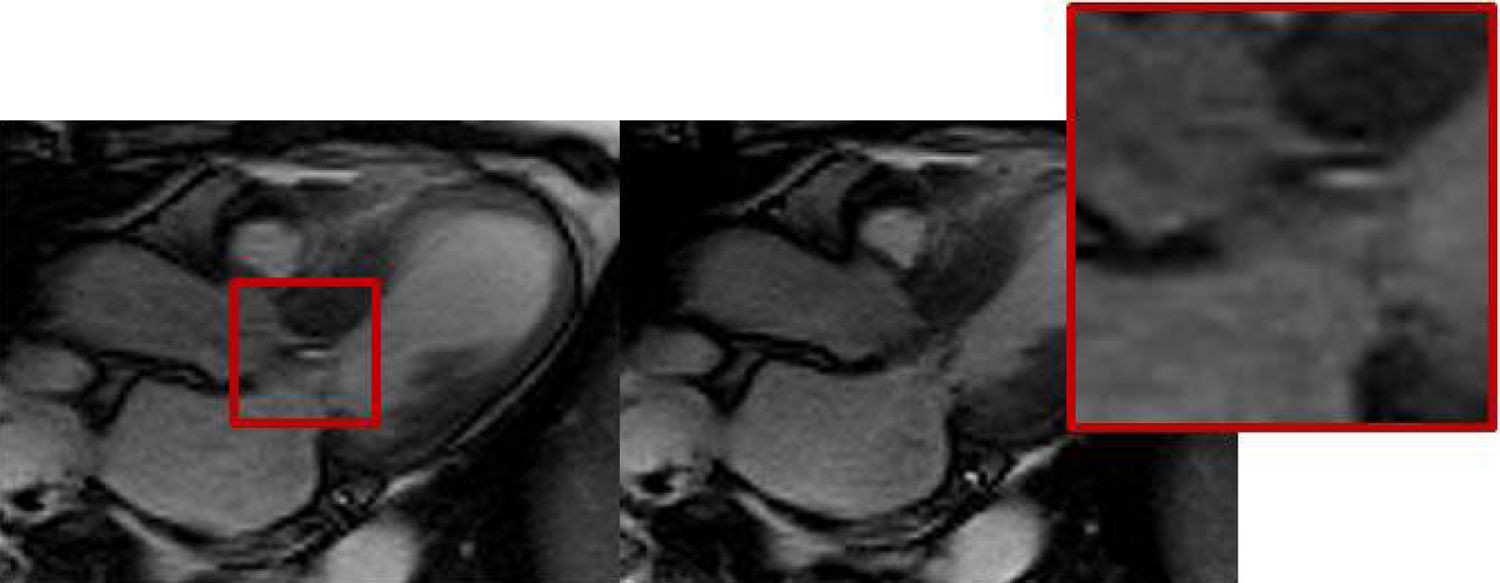
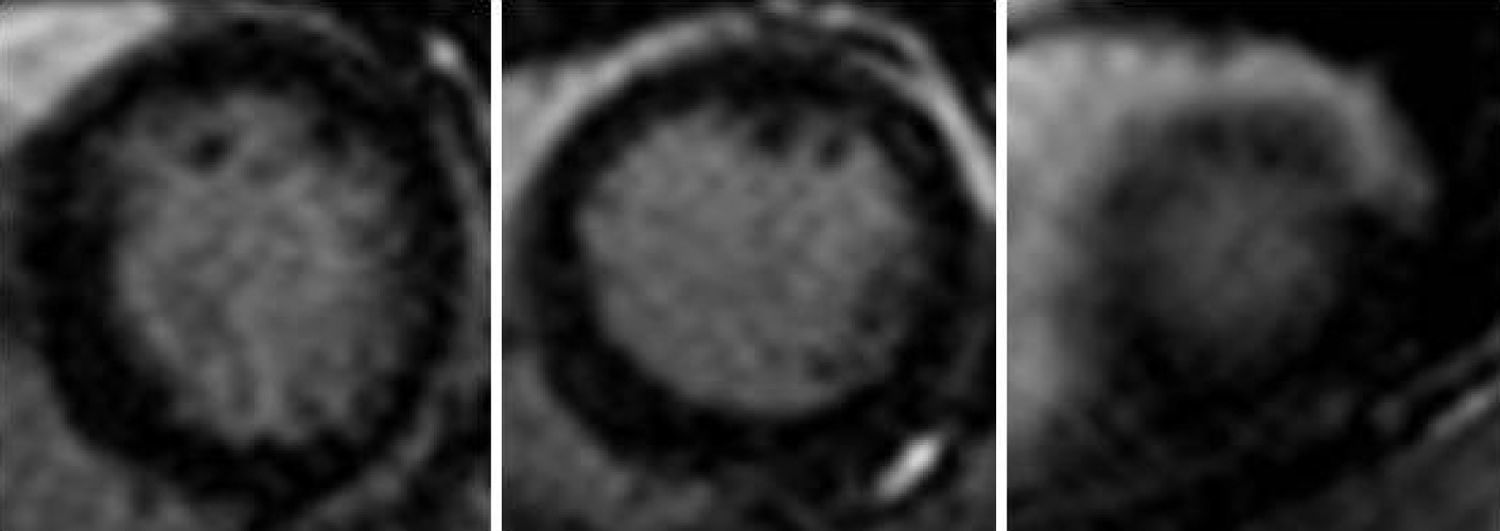
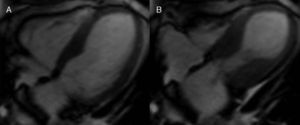
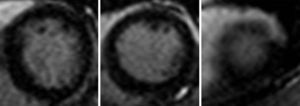
Cardiac MRI was ordered for further elucidation. The left ventricle showed apical ballooning with hypokinetic apex (Figure 3). Asymmetric hypertrophy of the LV walls was also noted, with mitral valve systolic anterior motion (SAM) and flow acceleration across the LV outflow tract (LVOT) (Figure 4). Delayed gadolinium myocardium enhancement was negative, excluding myocarditis and acute myocardial infarction (Figure 5).
A diagnosis of Takotsubo cardiomyopathy associated with asymmetric hypertrophic cardiomyopathy and LVOT obstruction was therefore made. After several days of observation and ambulation, the patient was discharged in a stable medical condition on aspirin, lisinopril and metoprolol.
DiscussionSince the original description by Tsuchihashi et al.1 in 2001, the pathophysiology of Takotsubo syndrome has not been fully established. The possible contribution of transient dynamic LVOT obstruction has been suggested from the beginning. Once present, the dynamic obstruction elevates left ventricular filling pressures, increasing myocardial oxygen demands and ultimately leading to apical hypoperfusion and ischemia. Some patients may have a geometric predisposition (sigmoid interventricular septum, small LVOT, reduced left ventricular volume) to dynamic LVOT obstruction, which may manifest only in a setting of intense adrenergic stimulation or hypovolemia.2,3
On the other hand, there are reports of residual SAM of the mitral apparatus without significant LVOT obstruction on the resting echocardiograms of patients two to four months after an episode of apical ballooning syndrome, the underlying SAM being indicated as the trigger for the further development of LVOT obstruction.4
Hence, considering this example, the causality dilemma of which came first, “the chicken or the egg”, naturally comes to mind.5
Recognition of acute dynamic LVOT obstruction as the initial mechanism in some patients with Takotsubo syndrome has important clinical and therapeutic implications, as the use of conventional treatment for acute chest pain and evidence of myocardial ischemia may include nitrates and afterload reduction, which would be likely to worsen the LVOT gradient, causing further clinical deterioration. The use of beta-blockers and intravenous fluids might be beneficial and even life-saving.2
Conflicts of interestThe authors have no conflicts of interest to declare.