The prevalence, complexity, clinical importance, heterogeneity and unpredictability of inherited cardiovascular diseases make the development of inherited cardiovascular disease centers an inevitability, with the ultimate goal of reducing the morbidity and mortality associated with these conditions. An inherited cardiovascular disease center may be seen as a subunit of a cardiology department, with health professionals specializing in these types of disorders, organized to provide excellence in all related areas, including diagnosis, treatment, follow-up, prevention, risk stratification and prognosis. Among its objectives are the development of action protocols and the creation of databases that enable patients to be included in national and international research networks. To achieve these objectives these centers should include functional units of clinical and basic sciences, research, training and education, acting in harmony in a holistic approach to patients and their families. As most experience on inherited cardiovascular diseases is based on hypertrophic cardiomyopathy and on “hypertrophic cardiomyopathy centers”, these centers represent an excellent opportunity to learn how to set up inherited cardiovascular disease centers. European centers will differ from country to country, reflecting the heterogeneity of national health systems, but will share a common core, presented in this document. Though we are aware that this ambitious project is not at all easy and may be difficult to implement in its entirety – in fact we consider it a major step – our position is that all the efforts to achieve it are worthwhile, considering that the main goal will always be the well-being of those affected by these particular disorders.
A prevalência, complexidade, importância clínica, heterogeneidade e imprevisibilidade das doenças cardíacas heredofamiliares torna a criação de Unidades de Doenças Cardíacas Hereditárias uma inevitabilidade, cujo objectivo final é reduzir a morbilidade e a mortalidade relacionadas com estas patologias. Uma Unidade de Doenças Cardíacas Hereditárias é uma subunidade de um departamento de Cardiologia constituída por profissionais de saúde especializados neste tipo de doenças, organizado de forma a proporcionar a excelência em todos as suas áreas, incluindo diagnóstico, tratamento, seguimento, prevenção, estratificação de risco e determinação de prognóstico. Alguns dos seus objectivos são o desenvolvimento de protocolos de actuação e a criação de bases de dados que permitam a inclusão de doentes em registos e redes de investigação nacionais e internacionais. Para atingir estes objectivos estes centros devem integrar Unidades Funcionais (clínica, ciências básicas, investigação, treino e educação), actuando harmonicamente numa abordagem holística de doentes e suas famílias. Como grande parte do conhecimento referente às doenças cardíacas hereditárias se baseia na miocardiopatia hipertrófica e na experiência adquirida com os “centros de miocardiopatia hipertrófica”, estes representam um excelente modelo para aprender como criar e desenvolver as Unidades de Doenças Cardíacas Hereditárias. A nível Europeu, as características destas Unidades serão diferentes de um país para outro, reflectindo a heterogeneidade dos diferentes sistemas e serviços nacionais de saúde, compartilhando no entanto um core comum, apresentado neste documento. Embora conscientes de que este projecto é ambicioso e de que a sua concretização como um todo não será uma tarefa fácil, consideramos a aplicação e divulgação deste conceito a nível nacional e Europeu um passo de grande importância. Assim, a nossa posição é que todos os esforços para atingir esta meta valem a pena, considerando que o principal objectivo será sempre o bem-estar dos doentes afectados por estas patologias.
Many of the inherited cardiovascular diseases are uncommon, but collectively they represent a substantial burden of disease, causing sudden cardiac death, heart failure and thromboembolism in many young people and adults in Europe every year. Many of these deaths can be prevented if timely diagnosis and expert management of these patients become standard practice across the continent.
Recent knowledge of the genetic basis of many of these conditions represents a turning-point in our approach, enabling their detection in asymptomatic or mildly symptomatic individuals. With genetic testing, although it may not be possible to prevent all sudden cardiac deaths caused by inherited diseases, many families could be spared the trauma of multiple deaths among their members. Moreover, the use of genetic tests (as opposed to conventional clinical surveillance) can reduce costs, because family members who do not carry the mutation can be discharged from follow-up.
Due to its higher prevalence and media impact, for a long time attention has been mainly focused on hypertrophic cardiomyopathy (HCM) and on HCM centers, but the general trend today is towards the development of inherited cardiovascular disease (ICVD) centers, specializing in a range of disorders rather than in a single one, with higher benefit-to-cost ratios.
European ICVD units will differ in each country, reflecting the heterogeneity of national health systems, but they should share a common core, as suggested in this document.
The unifying theme for these ICVD centers is the need for an accurate genetic diagnosis that, along with thorough cardiological investigation, will help provide management, counseling and advice for patients and their families. This requires not only the integration of cardiology and genetics but also the development of shared care between cardiological and non-cardiological departments, with appropriate referrals from primary care.
Five main categories of inherited cardiovascular conditions can be considered1:
- 1.
Arrhythmic syndromes (long and short QT syndromes, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia)
- 2.
Cardiomyopathies (hypertrophic, dilated, restrictive, others)
- 3.
Arteriopathies (Marfan, Ehlers-Danlos, others)
- 4.
Muscular dystrophies (Emery-Dreifuss muscular dystrophy, myotonic dystrophy, others)
- 5.
Familial hypercholesterolemia.
The ICVD centers would only include categories 1 to 4, as familial hypercholesterolemia is usually managed in specialist lipid clinics, with specific organizational and support arrangements.
The paradigm of hypertrophic cardiomyopathyMost of the experience on inherited cardiovascular diseases is centered on hypertrophic cardiomyopathy and on so-called “hypertrophic cardiomyopathy centers”.
As HCM is the most common and best-studied inherited cardiovascular disease, sharing most of the problems faced by other inherited heart conditions, HCM centers represent an excellent case-study to learn how to set up inherited cardiovascular disease centers. A thorough knowledge of HCM and its centers is thus essential to this subject.
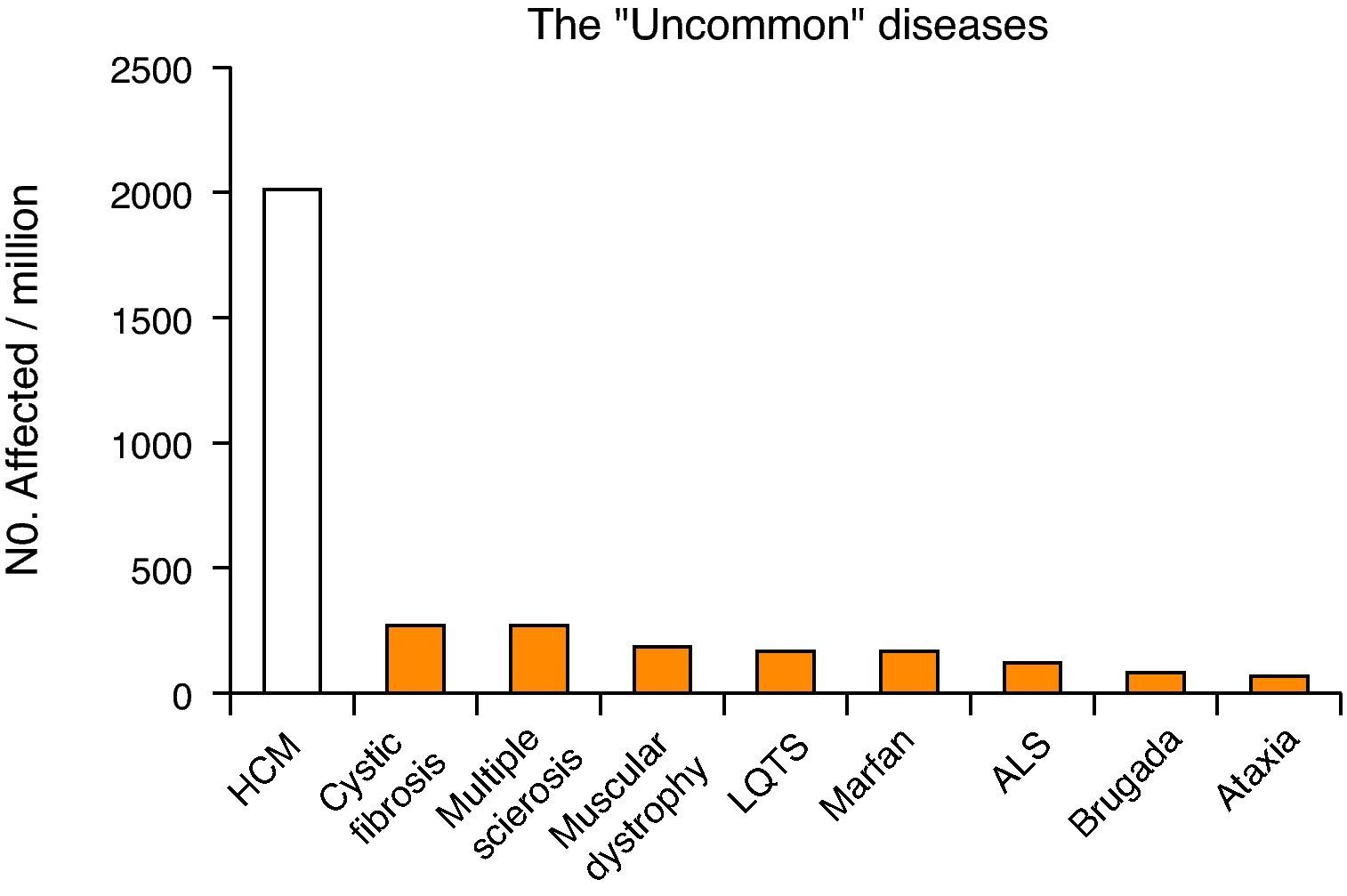
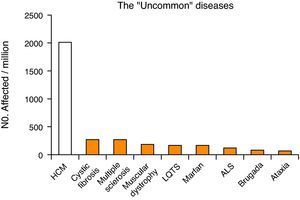
Hypertrophic cardiomyopathy has a prevalence of about 0.2%2 (1 in 500 individuals), and is thus much more common than other chronic diseases (including cystic fibrosis, multiple sclerosis, amyotrophic lateral sclerosis and Marfan syndrome) (Fig. 1). Unlike HCM, these diseases are classified as rare (defined as affecting less than 1 in 2000 individuals), with a special status, including specific institutional cooperation and support3,4. However, although not having the status of a rare disease, HCM patients face many of the difficulties associated with these conditions, such as delayed diagnosis, lack of prospective evaluation of the effectiveness of different therapeutic modalities, and limited access to advanced diagnosis and treatment methods.
The first HCM center was established nearly 20 years ago at the Royal Prince Alfred Hospital in Sydney, Australia. Later, other centers were opened in Europe (Hammersmith Hospital, London, UK) and in the United States (NIH, Bethesda, Maryland) 5. At present, there are numerous HCM centers, in North America (US and Canada), Europe (Italy, Germany, Ireland), the Middle East (Israel) and Asia (China). Currently, some of the main centers are located in Minneapolis (USA), London (UK) and Italy.
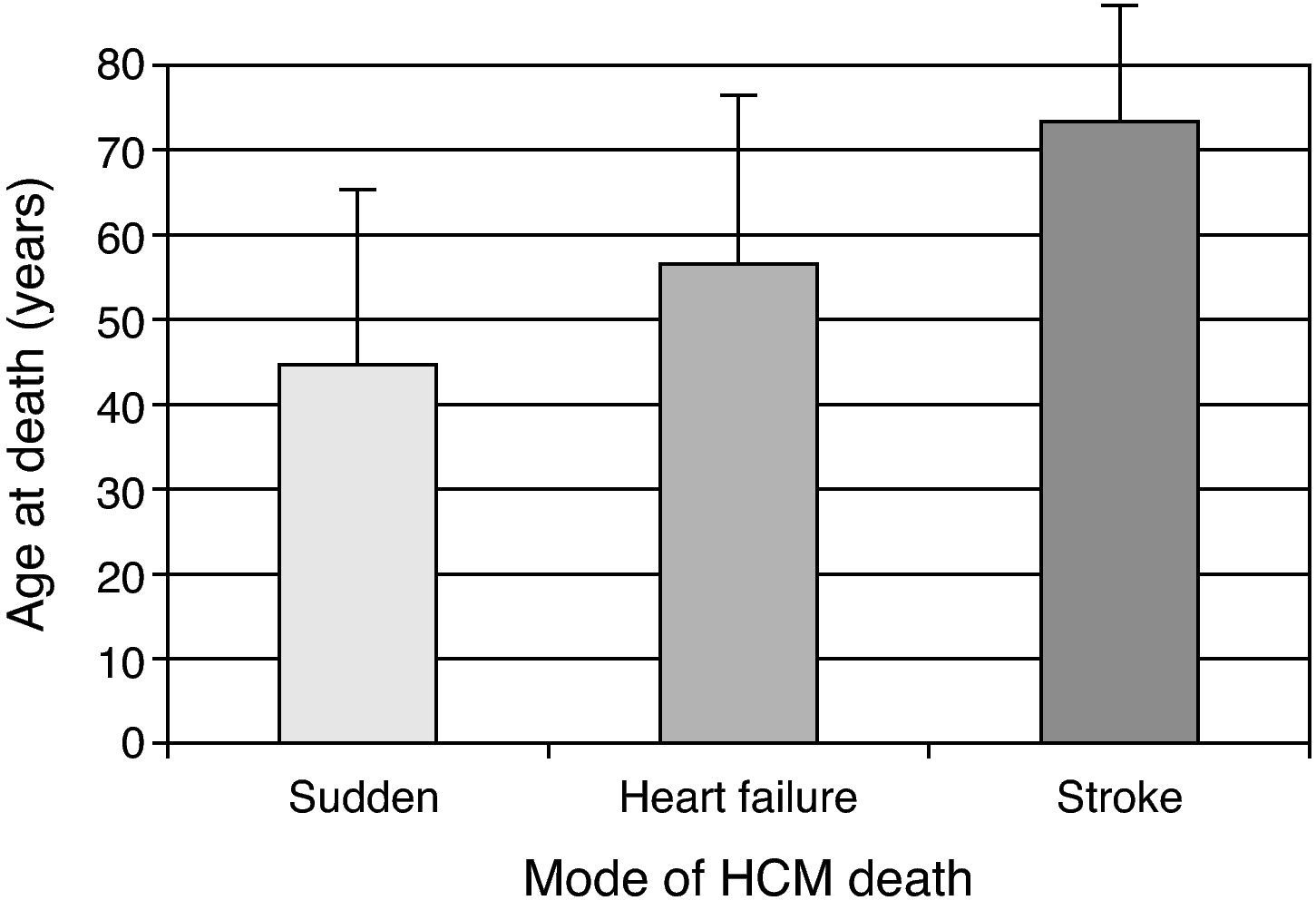
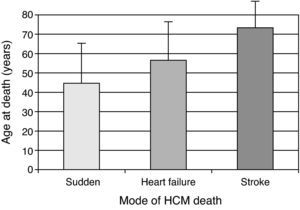
The clinical importance of HCM stems from two facts: it is a major cause of sudden death – an incidence of about 0.5% per year, versus about 0.1% per year in the general population – particularly in young populations; secondly, the disease is an important cause of heart failure and thromboembolic phenomena associated with significant morbidity and mortality, more frequently in older patients (Fig. 2) 6,7.
Mode of HCM death according to age.3
In 2009, using a definition that includes the whole of the transcontinental countries of Russia and Turkey8, the population of Europe was about 830.4 million (slightly more than 13% of the world population). The exact figure depends on the definition of the geographic extent of Europe, as in 2008 the population of the EU was 499 million, non-EU countries in Europe accounted for another 94 million, and five transcontinental countries totaled 240 million people, half of whom reside in Europe8,9.
On the basis of the prevalence mentioned above, there may be about 1660000 HCM patients in Europe. Of these, assuming that only 10-20% (166000 to 332000) are diagnosed, about 1328000 to 1494000 HCM patients are still undiagnosed (80-90% of the HCM population) and are therefore without the benefit of preventive and/or therapeutic measures to avoid sudden death or other complications of the disease.
Considering only this group of undiagnosed patients, between 6640 to 7470 patients (0.5% per year) will die suddenly every year in Europe without even knowing they have the disease and with no prior medical intervention. Moreover, these calculations can (and should) also be applied to individual countries (Table 1). Using the above figures for HCM prevalence and sudden death incidence, it can be estimated that a country with a relatively small population (10 million) will have about 20 000 inhabitants with HCM, most of them undiagnosed, with more than 75 patients per year at risk of sudden cardiac death, with no prior diagnosis or access to risk stratification and prevention. Applying these calculations to countries with much larger populations (Table 1) the tremendous impact of failure to diagnose this disease becomes obvious.
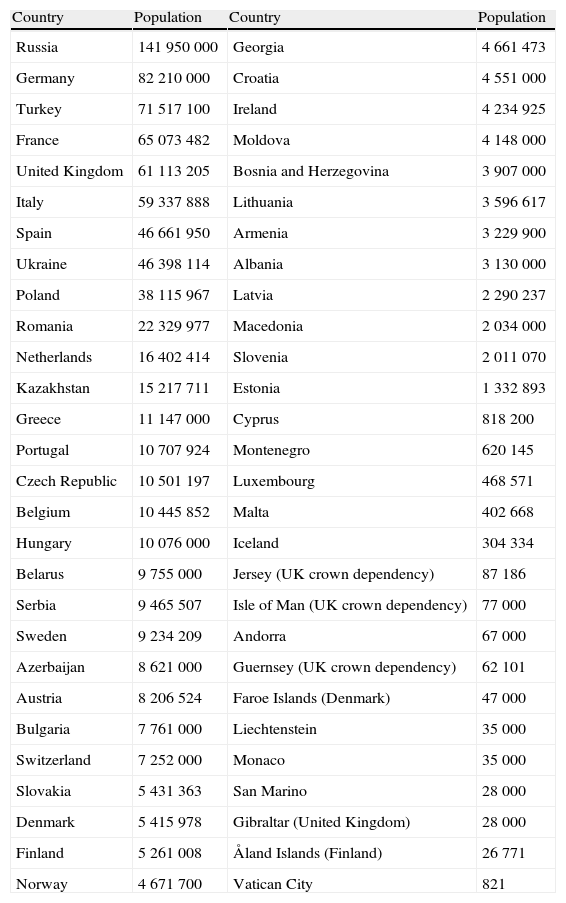
Population of European countries (http://pt.wikipedia.org/wiki/Europe)
| Country | Population | Country | Population |
| Russia | 141 950 000 | Georgia | 4 661 473 |
| Germany | 82 210 000 | Croatia | 4 551 000 |
| Turkey | 71 517 100 | Ireland | 4 234 925 |
| France | 65 073 482 | Moldova | 4 148 000 |
| United Kingdom | 61 113 205 | Bosnia and Herzegovina | 3 907 000 |
| Italy | 59 337 888 | Lithuania | 3 596 617 |
| Spain | 46 661 950 | Armenia | 3 229 900 |
| Ukraine | 46 398 114 | Albania | 3 130 000 |
| Poland | 38 115 967 | Latvia | 2 290 237 |
| Romania | 22 329 977 | Macedonia | 2 034 000 |
| Netherlands | 16 402 414 | Slovenia | 2 011 070 |
| Kazakhstan | 15 217 711 | Estonia | 1 332 893 |
| Greece | 11 147 000 | Cyprus | 818 200 |
| Portugal | 10 707 924 | Montenegro | 620 145 |
| Czech Republic | 10 501 197 | Luxembourg | 468 571 |
| Belgium | 10 445 852 | Malta | 402 668 |
| Hungary | 10 076 000 | Iceland | 304 334 |
| Belarus | 9 755 000 | Jersey (UK crown dependency) | 87 186 |
| Serbia | 9 465 507 | Isle of Man (UK crown dependency) | 77 000 |
| Sweden | 9 234 209 | Andorra | 67 000 |
| Azerbaijan | 8 621 000 | Guernsey (UK crown dependency) | 62 101 |
| Austria | 8 206 524 | Faroe Islands (Denmark) | 47 000 |
| Bulgaria | 7 761 000 | Liechtenstein | 35 000 |
| Switzerland | 7 252 000 | Monaco | 35 000 |
| Slovakia | 5 431 363 | San Marino | 28 000 |
| Denmark | 5 415 978 | Gibraltar (United Kingdom) | 28 000 |
| Finland | 5 261 008 | Åland Islands (Finland) | 26 771 |
| Norway | 4 671 700 | Vatican City | 821 |
Although the concept of centers of reference, or centers of expertise, is somewhat ill-defined, they may be described as the ideal places for referring particular groups of patients. These centers centralize both expertise and services, bringing together a group of multidisciplinary hospital-based competences, organized around highly specialized medical teams.
The ability to provide a comprehensive and effective service is grounded firstly in the individual expertise and experience of the professionals involved; secondly in easy access to specialized research; thirdly in their coming together as a multidisciplinary team; and finally, in the effectiveness of the team's working organization in delivering a highly specific care program. The creation of such a center requires more than simply a collection of experts from different disciplines. Rather, these centers should ensure that the needs of the whole population with regard to a specific condition are met and that patients and families are managed holistically, with the most efficient and effective use of resources10,11.
As agreed by international institutions1,10,11, the following eight criteria should define a center of reference/expertise:
- 1.
appropriate capacities to diagnose, follow and manage patients, with evidence of good outcomes;
- 2.
attractiveness, measured through volume of activity, which needs to be significantly larger than anticipated from the prevalence of the diseases in question and the catchment area (defined as the geographical area served by the hosting hospital for non-rare diseases) or national coverage;
- 3.
the ability to provide expert advice on diagnosis and management;
- 4.
the ability to produce and adhere to good practice guidelines and to implement outcome measures and quality control;
- 5.
demonstration of a multidisciplinary approach;
- 6.
a high level of expertise and experience documented through publications, grants or honorary positions, teaching and training;
- 7.
a strong contribution to research;
- 8.
close links and collaboration with other expert centers at national and international level and the ability to network.
The advantage of these centers lies in the fact that they not only provide a rating scheme that enables patients to identify the appropriate health care resources for their disease but also enable health care managers to identify where best to allocate specific financial resources in order to support the additional activities linked to their duties.
Rationale for the creation of ICVD centersThere are four major issues that increase the need for ICVD centers:
First, as the number of patients with inherited cardiovascular diseases is relatively small compared to the majority of patients seen in general cardiology consultations (most of whom have coronary, hypertensive or valvular heart disease), the experience of most clinical cardiologists in these diseases is relatively limited. The general trend is thus for knowledge of these common diseases to be extrapolated to the management of patients with ICVD, an approach that is often unsuitable and sometimes actually dangerous, given the specificity and complexity of the problems faced by those with these conditions. Second, as research in this area is in constant and rapid evolution, the non-expert in ICVD is often confused by the flood of data coming from the medical literature. Third, the complex pathophysiology of ICVD requires a wide range of knowledge and cooperation between different scientific areas and medical subspecialties, including genetics, molecular biology, heart failure, arrhythmias, myocardial ischemia, valvular disease, thromboembolism, cardiac imaging, hemodynamics, interventional cardiology, and cardiac surgery. Finally, there is a crucial need to standardize diagnostic and therapeutic approaches through the development of protocols and the creation of databases, which are essential for the development of research projects.
Inherited cardiovascular disease centers1,10,11An ICVD center may be considered a subunit of a cardiology department composed of clinicians and health professionals experienced in ICVD, organized to ensure an approach of excellence, according to the state of the art, in all areas of these diseases. This approach should cover diagnosis, treatment, follow-up, prevention, risk stratification and determination of prognosis, and ideally also include a strong component of basic and advanced research.
In these centers, patients with inherited cardiovascular conditions will be followed in specialized consultations with specific protocols, in which genotypic and phenotypic evaluation of the disease is performed, assessing not only the patient but also his or her family.
This type of systematic approach to specific diseases, concentrating large numbers of patients with the same disorder in referral centers, can have a significant impact on morbidity and mortality. This has been demonstrated for pulmonary hypertension and cystic fibrosis, in which increased survival and better quality of life for affected patients have been observed since this strategy was implemented throughout the world3,4.
ObjectivesThe main objectives of ICVD centers are:
- 1.
to provide clinical activity of excellence, according to the state of the art, to ICVD patients and their families (including the above-mentioned close interaction between cardiology and genetics);
- 2.
to create core research in ICVD;
- 3.
to train new clinicians and researchers with special expertise in ICVD;
- 4.
to contribute to education and awareness concerning ICVD in the scientific community, society, government, and the pharmaceutical and device industry, in order to disseminate knowledge of the diseases and their multiple and complex problems, with the objective of obtaining institutional and non-institutional support for patients and of developing credible research projects in this area.
Organization is a key issue in achieving the above objectives.
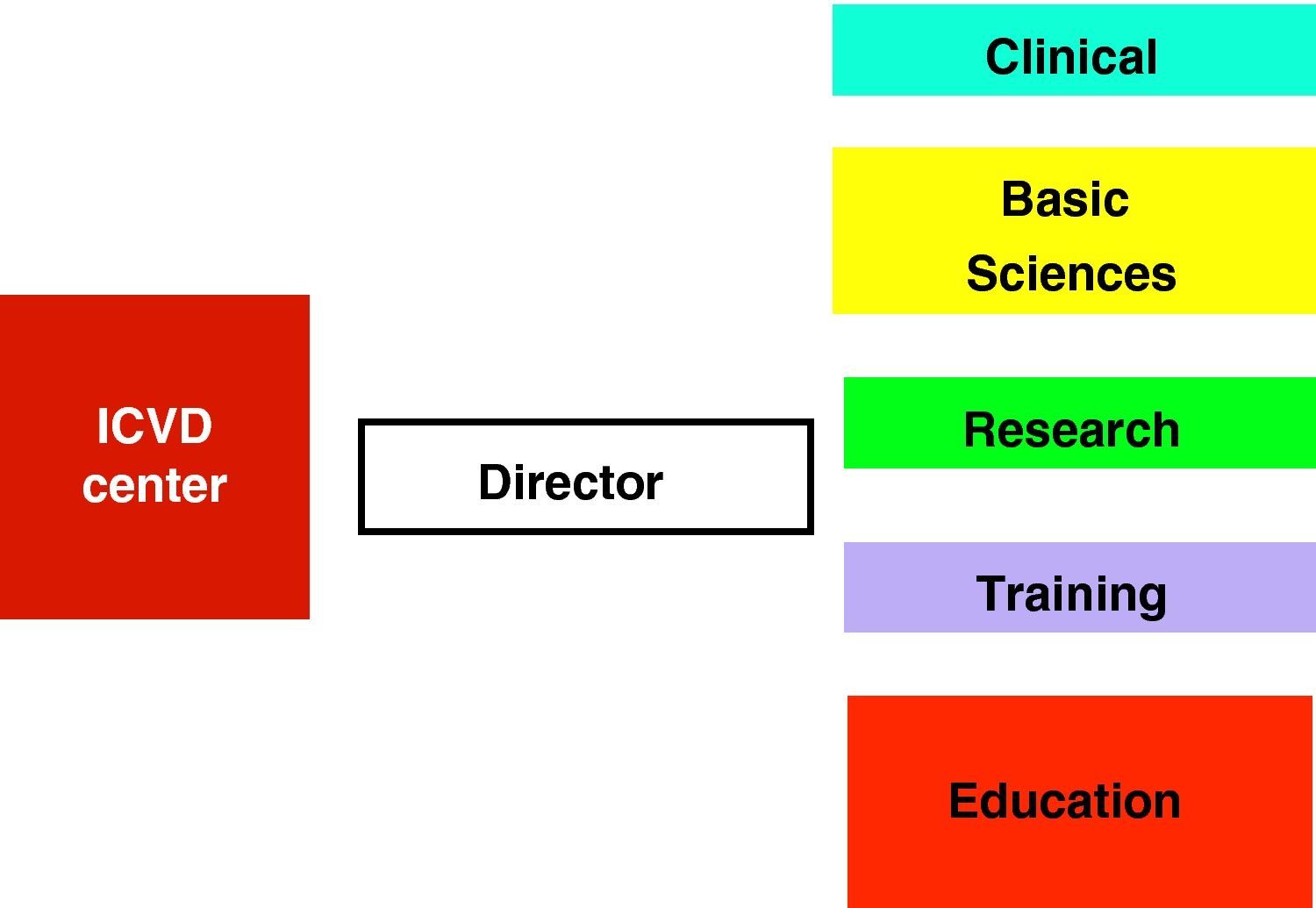
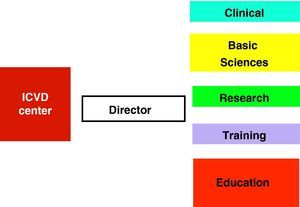
An ICVD center should have a director and five functional units (clinical, basic sciences, research, training, and education and awareness) (Fig. 3), closely inter-related and with extensive overlapping areas. It should be located in a major tertiary center, and be accessible to all its catchment population. In small countries, such a center could be national in scope, with links to all regions of the country (with peripheral clinics or satellite centers).
- a)
Director: with clinical expertise in ICVD and a background in cardiac imaging;
- b)
Functional Units: each with specific subdivisions.
- a)
Multidisciplinary clinical team. This team will include a number of specialists from different areas, not only from clinical cardiology (adult and pediatric consultants) and genetics (clinical geneticists and genetic counselors) but also consultants from other medical specialties (including neurology, ophthalmology, internal medicine, psychiatry, and clinical psychology) and specialists from other areas (nutrition, nursing, and social support). As mentioned above, the interaction between cardiology and clinical genetics plays a key role in this unit. It is current practice, whenever possible, to perform genetic (rather than clinical) cascade screening, a cost-effective model which enables family members to be identified as not being at risk and so to be discharged from follow-up, or identified as mutation carriers and hence requiring follow-up.
- b)
Multimodality imaging and electrocardiology department with the ability to perform rest and exercise ECG, echocardiography, cardiac magnetic resonance imaging and cardiac computed tomography (with collaboration between cardiology and radiology teams).
- c)
Arrhythmology and electrophysiology department, meeting patients’ specific needs such as electrophysiological studies, ablation procedures or device implantation (pacemakers, resynchronization devices and implantable cardioverter-defibrillators).
- d)
Catheterization and interventional cardiology department, providing diagnostic data (such as coronary and aortic angiography) and therapeutic procedures (alcohol septal ablation, coronary intervention), working in concert with the cardiac surgery department.
- e)
Cardiac surgery department with the ability to provide all types of cardiovascular surgery, including major aortic surgery, myectomy and valve surgery.
An ICVD center should have easy access not only to a certificated laboratory or institute of molecular genetics and molecular biology but also to a cardiovascular pathology department. Ideally, the basic sciences unit should work in close cooperation with the clinical team.
Research unitThis functional unit will focus on basic, translational and clinical research, promoting the interface between clinical and basic sciences for appropriate translation of knowledge. Participation in clinical trials under strict ethical rules is desirable in an ICVD center. The high level of expertise and experience of the centers should be documented through publications, grants, honorary positions, teaching and training. Involvement in epidemiological surveillance such as registries is recommended. The unit should have the ability to produce and adhere to good practice guidelines. Cooperation between centers in different countries should be favored through European reference networks. All projects should be approved by local (institutional) ethics committees.
Training unitSharing of knowledge between and within different specialties is central to the aims of this unit. Providing training for health professionals should be a major goal, considering in particular the specific and often complex knowledge required in an ICVD center.
Education and awareness unitThe team should be aware of the importance of disseminating specific information to the general public, in order to raise awareness about the disease and its related problems and implications.
Types of approachAs indicated above, ICVD centers should adopt a global approach, not only to the patient but also to his or her family, considering the familial inheritance of ICVD and the various problems involved (clinical, social, legal, and psychological).
Individual approachIn ICVD centers two groups of patients are expected to be followed: those followed in the center, and patients followed in other institutions but occasionally referred to the ICVD center for a second opinion or to perform additional diagnostic tests.
Clinical evaluation of patients in the center will include genetic study and a broad phenotypic characterization with stratification of risk for sudden death every two years. It will also include education, genetic counseling and individualized therapy (including general measures, lifestyle, medical treatment, surgery, septal ablation, defibrillators and pacemakers).
Family-based approachThe ICVD center also has the task of studying the ICVD patient's family in “family consultations”, providing the logistical basis for a complete genotypic and phenotypic study of the whole family, in a comprehensive approach. Cascade screening (genetic and/or clinical) appears to be the most cost-effective approach12. Special attention should be paid to the possible need for psychological support and to the state of mutation carriers.
Organizational models1,10,11It is important to realize that not all specialized groups should expect to establish an ICVD center. It is unlikely that small clinics or departments could develop the necessary critical mass to gain sufficient experience of these conditions and to justify the investment in professional training (medical, pathology, genetic counseling, specialist nursing, laboratory, and cardiac physiology) and organizational structures to develop into a fully comprehensive service.
The most successful organizational model is the “hub and spoke” model. In this model, referrals coming from the periphery (spokes) are directed to the specialist tertiary service (hub). Once patients have been assessed by the hub, much of the patient care may be returned to the spokes according to agreed care guidelines.
Another possibility is the “specialist and satellite” model. In this model specialist centers have satellites in peripheral clinics to enable greater ease of access by patients who live far from the specialist center. Telemedicine may have an important role in this model.
Cooperative links1,10,11The ICVD center should propose the establishment of cooperation protocols with other institutions dealing with inherited cardiovascular diseases, including working groups on myocardial and pericardial diseases of national cardiology societies, national institutes of forensic and sports medicine, universities and colleges (schools of medicine, pharmacology, etc.), the Working Group on Myocardial and Pericardial Diseases of the European Society of Cardiology, other national and international ICVD centers, and other entities connected in any way with ICVD, such as patient support groups.
ConclusionsThe prevalence, complexity, clinical importance, heterogeneity and unpredictability of inherited cardiovascular diseases make the development of ICVD centers an inevitability, with the ultimate goal of reducing the morbidity and mortality associated with these conditions. An ICVD center may be seen as a subunit of a cardiology department, with health professionals specializing in these types of disorders, organized to provide excellence in all related areas, including diagnosis, treatment, follow-up, prevention, risk stratification and prognosis. Among its objectives are the development of action protocols and the creation of databases that enable patients to be included in national and international research networks. To achieve these objectives these centers should include functional units of clinical and basic sciences, research, training and education, acting in harmony in a holistic approach to patients and their families.
As most experience on inherited cardiovascular diseases is based on hypertrophic cardiomyopathy and on “hypertrophic cardiomyopathy centers”, these centers represent an excellent opportunity to learn how to set up inherited cardiovascular disease centers.
European ICVD centers will differ from country to country, reflecting the heterogeneity of national health systems, but will share a common core presented in this document. Though we are aware that this ambitious project is not at all easy and may be difficult to implement in its entirety – in fact we consider it a major step – our position is that all the efforts to achieve it are worthwhile, considering that the main goal will always be the well-being of those affected by these particular disorders.
Conflicts of interestThe authors declare they have no conflicts of interest.