Assessment of ischemic and bleeding risk is critical for the management of elderly patients with acute coronary syndromes, but it has been little studied.
ObjectiveThis study aims to assess the applicability of the GRACE and CRUSADE scores in patients aged ≥80 years with non-ST-elevation acute coronary syndrome (NSTE-ACS), and to identify the main predictors of in-hospital mortality and major bleeding in this population.
MethodsWe analyzed 544 patients aged ≥80 years with NSTE-ACS included in the Portuguese Registry on Acute Coronary Syndromes and identified the predictors of in-hospital mortality and major bleeding during hospitalization. Prediction models were created for these endpoints, then compared with the GRACE and CRUSADE scores, and their applicability to the study population was assessed.
ResultsUse of coronary angiography was associated with reduced risk of in-hospital mortality, without increasing risk of major bleeding (OR 0.2, 95% CI 0.006–0.49, p=0.001). Major bleeding was an independent predictor of in-hospital mortality (OR 10.9, 95% CI 2.36–50.74, p=0.002), and was associated with comorbidities and pharmacological therapy during hospitalization. The GRACE score showed good diagnostic accuracy for in-hospital mortality (AUC 0.75, 95% CI 0.63–0.87, p<0.001), but the CRUSADE score had weak discriminatory capacity for major bleeding (AUC 0.51, 95% CI 0.30–0.63, p=0.942), unlike our prediction model (AUC 0.68, 95% CI 0.52–0.84, p=0032).
ConclusionsThe GRACE score is suitable for risk assessment in octogenarians with NSTE-ACS, but the CRUSADE score is inadequate, and new scores are required to assess bleeding risk in this age-group.
A avaliação do risco isquémico e hemorrágico é fundamental na abordagem dos idosos com síndromes coronárias agudas, mas tem sido pouco estudada.
ObjetivoEste estudo pretende avaliar a adequação dos scores GRACE e CRUSADE a doentes com síndrome coronária aguda sem supradesnivelamento-ST e idade ≥80 anos, e identificar os principais preditores de mortalidade intra-hospitalar e hemorragia major nesta população.
MétodosForam avaliados 544 doentes com idade ≥80 anos com síndrome coronária aguda sem supradesnivelamento-ST, incluídos no Registo Português de Síndromes Coronárias Agudas. Foram identificados os preditores de mortalidade intra-hospitalar e de hemorragia major durante o internamento. Criaram-se modelos preditores destes endpoints, posteriormente comparados com os scores GRACE e CRUSADE, e avaliada a sua adequação à população em estudo.
ResultadosA realização de coronariografia associou-se a redução do risco de mortalidade intra-hospitalar, sem aumento do risco de hemorragia major (OR 0,2, IC 95% 0,006-0,49, p=0,001). A hemorragia major foi preditora independente de mortalidade intra-hospitalar (OR 10,9, IC 95% 2,36-50,74, p=0,002), e associou-se a comorbilidades e à terapêutica farmacológica instituída. O score GRACE apresentou boa acuidade diagnóstica para mortalidade intra-hospitalar (AUC 0,75, IC 95% 0,63-0,87, p<0,001), mas o CRUSADE mostrou fraca capacidade discriminatória de hemorragia major (AUC 0,51, IC 95% 0,30-0,63, p=0,942), contrariamente ao modelo preditor (AUC 0,68, IC 95% 0,52-0,84, p=0,032).
ConclusõesO score GRACE é adequado para avaliação de risco nos octogenários, mas o CRUSADE é desajustado, sendo necessários novos scores para a avaliação de risco hemorrágico nesta faixa etária.
acute coronary syndrome
area under the curve
coronary artery disease
chronic obstructive pulmonary disease
in-hospital mortality
major bleeding
myocardial infarction
non-ST-elevation acute coronary syndromes
non-ST-elevation myocardial infarction
prediction model
Portuguese Registry on Acute Coronary Syndromes
unstable angina
Invasive treatment strategies, together with aggressive antithrombotic medication, have been shown to reduce ischemic complications in patients with non-ST-elevation acute coronary syndromes (NSTE-ACS), although at the cost of an increase in bleeding complications.1–4 Weighing the risks and benefits of these therapies is crucial for reducing mortality in these patients, and the European Society of Cardiology (ESC) currently recommends the use of scores to stratify ischemic risk, such as the GRACE score,5 and bleeding risk, such as the CRUSADE score.6
Advanced age is associated with greater prevalence and extent of coronary artery disease (CAD) and higher risk of ischemic complications and mortality; 30% of deaths related to myocardial infarction (MI) occur in patients aged over 85,7 and most deaths in patients aged ≥75 years are of ischemic origin.8 Given their increased risk, elderly patients would in theory benefit more from therapies that improve outcomes.9–11 However, the particular characteristics of this age-group – presence of multiple comorbidities, reduced ability to perform activities of daily living, and physical, psychological and cognitive frailty – affect prognosis after NSTE-ACS and limit the benefit of aggressive therapies.11 Furthermore, drug metabolism is altered in the elderly, due to deterioration of renal and liver function, interactions caused by multiple medications, changes in body composition and comorbidities. These factors make older patients more likely to suffer adverse effects of therapy and are responsible for the significantly higher incidence of bleeding complications in this age-group.11 As a result, physicians are wary of using invasive treatments and aggressive antithrombotic medication in the elderly, despite their potential benefit. This is reflected in the results of various series that show a low rate of use of such therapies in older age-groups, even in the absence of contraindications.10,11
Notwithstanding the increased complexity of management in elderly patients with NSTE-ACS, data on those aged ≥80 years are limited, since such patients are frequently excluded from clinical trials.7,11 Current guidelines for this population are thus based on extrapolation of data for younger patients, which may not be applicable to such advanced ages. Specifically, it is not known whether the scores recommended for assessing ischemic and bleeding risk are appropriate for this age-group.
It is thus important to obtain information from other sources, such as registries. The Portuguese Registry on Acute Coronary Syndromes (ProACS)12 is sufficiently robust to assess this population, as shown by other studies based on its results.13,14
The main aims of this study are to assess the applicability of the GRACE and CRUSADE scores to patients aged ≥80 years with NSTE-ACS, and to identify the main predictors of in-hospital mortality (IHM) and major bleeding (MB) in this population.
MethodsStudy designIn this multicenter observational study of patients prospectively enrolled in the second phase of the ProACS13 with a diagnosis of NSTE-ACS and aged ≥80 years, predictors of IHM and in-hospital MB were analyzed and prediction models (PM) were created for these endpoints. The accuracy of the GRACE and CRUSADE scores and the prediction models for IHM (IHM-PM) and MB (MB-PM) were then compared.
Patient selectionWe analyzed 544 consecutive patients aged ≥80 years diagnosed with NSTE-ACS in 14 Portuguese hospitals and included in the ProACS between October 1, 2010 and October 25, 2012.
NSTE-ACS was defined on the basis of an admission diagnosis of non-ST-elevation myocardial infarction (NSTEMI), defined as lack of persistent ST elevation (<30 min) associated with elevated cardiac biomarkers (troponin or CK-MB) in a clinical context suggestive of myocardial ischemia, or unstable angina (UA), defined as angina or anginal equivalent, with or without ECG changes and without elevated cardiac biomarkers.
The ProACS12 is an observational study with continuous and prospective inclusion of all adult patients (≥18 years) diagnosed with acute coronary syndrome (ACS) within 48 hours of symptom onset. MI types 2, 4 and 5 according to the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction (2007)15 were excluded. Further information on the registry protocol is available on the website of the Portuguese Society of Cardiology.16
Data collectionThe following variables from the ProACS were included in the univariate analysis: demographic data (age, gender, body mass index [BMI]); cardiovascular risk factors (documented history of hypertension, diabetes, dyslipidemia, current smoking in the 30 days before admission, family history of CAD); personal cardiovascular (MI, heart failure [HF], stroke/transient ischemic attack) and non-cardiovascular history (cancer, clinically significant or life-threatening bleeding, dementia, chronic obstructive pulmonary disease [COPD], chronic renal disease [defined as previous history or presence of at least one of the following criteria: creatinine prior to admission >2 mg/dl, dialysis or kidney transplantation]); medication prior to admission (antiplatelets: aspirin, clopidogrel, both, or others; anticoagulants: vitamin K antagonists, others); data on hospital admission (location, mode of transport to the hospital, symptoms, blood pressure, heart rate, Killip class,17 ST-segment depression on ECG); and laboratory test results (isolated troponin elevation, hemoglobin, HbA1c, hematocrit, platelets, blood glucose, and creatinine). Hematocrit was calculated on the basis of hemoglobin level in accordance with the formula of Bain and Bates18 (hematocrit=hemoglobin [g/dl]×3). Creatinine clearance was estimated using the Cockcroft-Gault formula.19 Data on hospital stay were also recorded, including duration, laboratory values (minimum hemoglobin, peak creatinine), medication (antiplatelets: aspirin, clopidogrel, others; anticoagulants: unfractionated heparin, enoxaparin, fondaparinux, vitamin K antagonists, others; glycoprotein IIb/IIIa inhibitors, beta-blockers, angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs], statins, nitrates, calcium channel blockers, ivabradine, aldosterone antagonists, diuretics, antiarrhythmics, insulin, oral antidiabetic agents, inotropic agents, levosimendan), whether coronary angiography was performed, vascular access, use of vascular closure devices, culprit vessel, number of vessels with ≥50% stenosis, percutaneous coronary intervention (PCI), type of stent used, coronary artery bypass grafting (CABG), left ventricular (LV) function, temporary pacing, intra-aortic balloon pump, and invasive and non-invasive mechanical ventilation, as well as in-hospital complications (reinfarction, mechanical complications, arrhythmias, resuscitation following cardiac arrest, stroke, HF, major bleeding and death).
The GRACE5 and CRUSADE6 scores were calculated for all patients.
Further information on the definition of these variables is available on the website of the Portuguese Society of Cardiology.20
Study endpointsThe study endpoints were IHM and MB.
IHM was defined as death from any cause during hospitalization for NSTE-ACS. MB was defined as intracranial bleeding or bleeding resulting in hemodynamic compromise requiring treatment, the criteria in the Global Use of Strategies to Open Occluded Arteries (GUSTO) trial.21 The GUSTO criteria were chosen because this classification is used in the ProACS; the authors also considered it the most appropriate for the population and the study objectives, since it was important to identify bleeding that was sufficiently severe to justify not using therapies which reduce ischemic complications but increase bleeding risk in ACS.
Statistical analysisAn overall descriptive analysis of the study population was performed based on all the parameters assessed. Categorical variables were expressed as absolute and relative frequencies, and continuous variables as means and standard deviation.
Independent predictors of each of the study endpoints, IHM and MB, were identified, using univariate analysis to test the association between the endpoint and each potential predictor. The chi-square test was used for categorical variables and the t test for continuous variables. The Mann-Whitney test was used when the assumptions for the t test were not met. The normality of the distribution of continuous variables and their equality of variances were determined by the Kolmogorov-Smirnov test and the Levene test, respectively.
Logistic regression techniques were applied to identify independent predictors of IHM and MB considering all variables with statistical significance, using the forward stepwise method with likelihood ratios. The model included variables used in the CRUSADE score and, separately, variables related to medication during hospitalization: dual antiplatelet therapy, dual antiplatelet therapy plus anticoagulants (triple therapy), or dual antiplatelet therapy plus glycoprotein IIb/IIIa inhibitors (triple antiplatelet therapy). The entry and exit values were set at 0.10 and 0.15, respectively. The risk for each endpoint associated with each predictor was estimated using odds ratios and 95% confidence intervals (CI).
On the basis of the logistic regression analysis (“Enter” method), prediction models for IHM and MB were created on the basis of variables at admission with statistical significance for each endpoint or clinical significance: IHM-PM (LV ejection fraction <30%, Killip class >I, ST-segment depression on ECG, systolic blood pressure, heart rate, blood glucose, creatinine, and isolated troponin elevation) and MB-PM (history of bleeding, history of COPD, Killip class at admission >I, and admission creatinine). The fit of the models was assessed using the Hosmer-Lemeshow test.
On the basis of the regression coefficients, the predictive value of each model was determined and its discriminatory capacity was estimated by calculating the area under the receiver operating characteristic (ROC) curve, together with its sensitivity and specificity. ROC curves evaluate the ability of a model to attribute a higher probability of events to patients who suffered the study endpoints. In this way the diagnostic accuracy of the GRACE score and the IHM-PM for IHM, and the CRUSADE score and the MB-PM for MB, were assessed, using MedCalc for Windows version 9.2.0.1. The rest of the statistical analysis was performed with SPSS version 19, using the Hanley-McNeil test. The level of significance was taken to be 5%.
ResultsCharacteristics of the study populationThe characteristics of the 544 patients studied are presented in Table 1. Ages ranged from 80 to 92 years (mean 84). Diagnosis at admission was NSTEMI in 91% (n=495) and UA in 9% (n=49). Figure 1 shows the distribution of the total population and of patients with IHM and MB according to the GRACE and CRUSADE scores, respectively. In the study population, 93.6% of patients who suffered IHM were classified as high risk by the GRACE score, while the CRUSADE score classified only 53.9% of those with MB as high or very high risk.
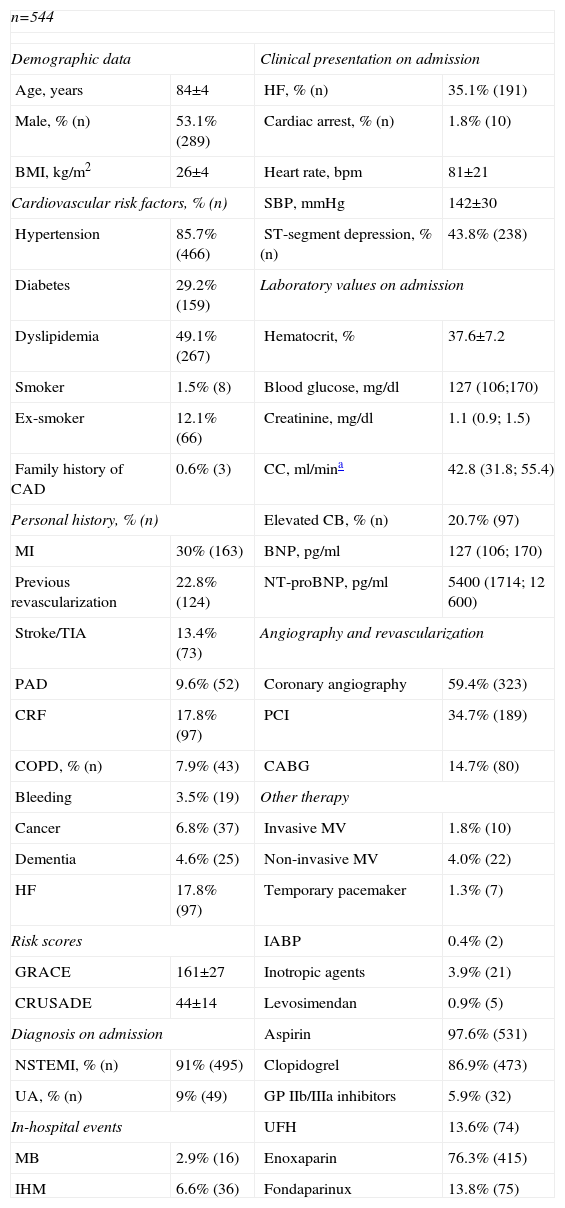
Baseline characteristics of the study population.
| n=544 | |||
| Demographic data | Clinical presentation on admission | ||
| Age, years | 84±4 | HF, % (n) | 35.1% (191) |
| Male, % (n) | 53.1% (289) | Cardiac arrest, % (n) | 1.8% (10) |
| BMI, kg/m2 | 26±4 | Heart rate, bpm | 81±21 |
| Cardiovascular risk factors, % (n) | SBP, mmHg | 142±30 | |
| Hypertension | 85.7% (466) | ST-segment depression, % (n) | 43.8% (238) |
| Diabetes | 29.2% (159) | Laboratory values on admission | |
| Dyslipidemia | 49.1% (267) | Hematocrit, % | 37.6±7.2 |
| Smoker | 1.5% (8) | Blood glucose, mg/dl | 127 (106;170) |
| Ex-smoker | 12.1% (66) | Creatinine, mg/dl | 1.1 (0.9; 1.5) |
| Family history of CAD | 0.6% (3) | CC, ml/mina | 42.8 (31.8; 55.4) |
| Personal history, % (n) | Elevated CB, % (n) | 20.7% (97) | |
| MI | 30% (163) | BNP, pg/ml | 127 (106; 170) |
| Previous revascularization | 22.8% (124) | NT-proBNP, pg/ml | 5400 (1714; 12 600) |
| Stroke/TIA | 13.4% (73) | Angiography and revascularization | |
| PAD | 9.6% (52) | Coronary angiography | 59.4% (323) |
| CRF | 17.8% (97) | PCI | 34.7% (189) |
| COPD, % (n) | 7.9% (43) | CABG | 14.7% (80) |
| Bleeding | 3.5% (19) | Other therapy | |
| Cancer | 6.8% (37) | Invasive MV | 1.8% (10) |
| Dementia | 4.6% (25) | Non-invasive MV | 4.0% (22) |
| HF | 17.8% (97) | Temporary pacemaker | 1.3% (7) |
| Risk scores | IABP | 0.4% (2) | |
| GRACE | 161±27 | Inotropic agents | 3.9% (21) |
| CRUSADE | 44±14 | Levosimendan | 0.9% (5) |
| Diagnosis on admission | Aspirin | 97.6% (531) | |
| NSTEMI, % (n) | 91% (495) | Clopidogrel | 86.9% (473) |
| UA, % (n) | 9% (49) | GP IIb/IIIa inhibitors | 5.9% (32) |
| In-hospital events | UFH | 13.6% (74) | |
| MB | 2.9% (16) | Enoxaparin | 76.3% (415) |
| IHM | 6.6% (36) | Fondaparinux | 13.8% (75) |
BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CB: cardiac biomarkers; CC: creatinine clearance; COPD: chronic obstructive pulmonary disease; CRF: chronic renal failure; GP: glycoprotein; HF: heart failure; IABP: intra-aortic balloon pump; IHM: in-hospital mortality; MB: major bleeding; MI: myocardial infarction; MV: mechanical ventilation; NSTEMI: non-ST-segment myocardial infarction; NT-proBNP: N-terminal pro-brain-type natriuretic peptide; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; TIA: transient ischemic attack; UA: unstable angina; UFH: unfractionated heparin.
Around 98% of patients were medicated with aspirin, 86.9% with clopidogrel and 5.9% with glycoprotein IIb/IIIa inhibitors. Enoxaparin was the most commonly prescribed anticoagulant. Coronary angiography was performed in 59.4% of patients, PCI in 34.7% and CABG in 14.7%. The mean GRACE score in this population was 161±27 and the mean CRUSADE score was 44±14. Median hospital stay was five days (25th percentile: 3; 75th percentile: 8); the IHM endpoint was observed in 6.6% and the MB endpoint in 2.9% (Table 1).
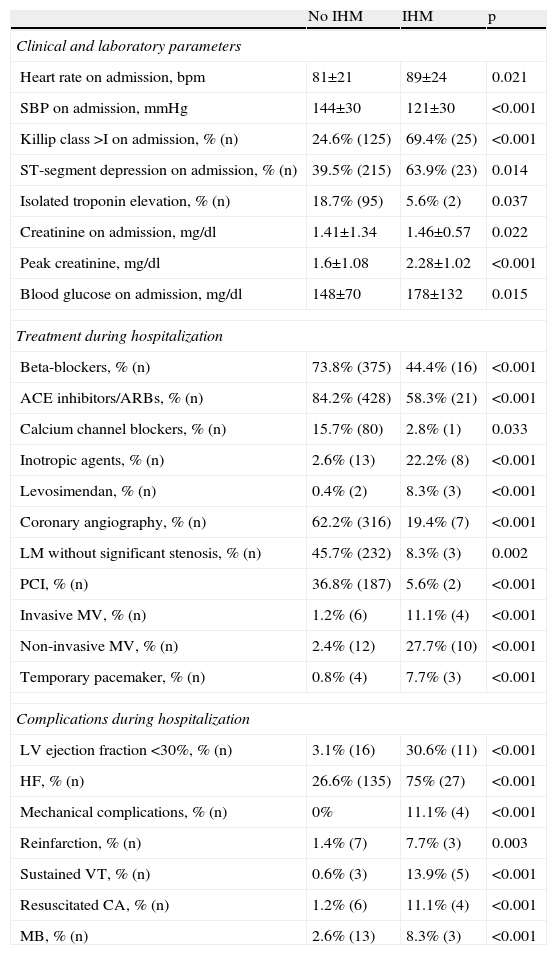
Predictors of in-hospital mortalityIn univariate analysis, IHM was associated with higher heart rate and creatinine on admission, peak creatinine, and Killip class >I, ST-segment depression, isolated troponin elevation, and lower systolic blood pressure on admission. In-hospital survival was associated with medication with beta-blockers, calcium channel blockers and ACE inhibitors/ARBs, coronary angiography and PCI, and absence of left main disease. Patients who died during hospitalization more frequently received levosimendan, inotropic agents, mechanical ventilation and temporary pacing, and had a higher prevalence of ischemic and bleeding complications (Table 2). No association was seen between IHM and age, cardiovascular risk factors, previous medication, vascular access or closure devices used in coronary angiography, type of stent used for PCI, or CABG.
Variables associated with in-hospital mortality on univariate analysis.
| No IHM | IHM | p | |
| Clinical and laboratory parameters | |||
| Heart rate on admission, bpm | 81±21 | 89±24 | 0.021 |
| SBP on admission, mmHg | 144±30 | 121±30 | <0.001 |
| Killip class >I on admission, % (n) | 24.6% (125) | 69.4% (25) | <0.001 |
| ST-segment depression on admission, % (n) | 39.5% (215) | 63.9% (23) | 0.014 |
| Isolated troponin elevation, % (n) | 18.7% (95) | 5.6% (2) | 0.037 |
| Creatinine on admission, mg/dl | 1.41±1.34 | 1.46±0.57 | 0.022 |
| Peak creatinine, mg/dl | 1.6±1.08 | 2.28±1.02 | <0.001 |
| Blood glucose on admission, mg/dl | 148±70 | 178±132 | 0.015 |
| Treatment during hospitalization | |||
| Beta-blockers, % (n) | 73.8% (375) | 44.4% (16) | <0.001 |
| ACE inhibitors/ARBs, % (n) | 84.2% (428) | 58.3% (21) | <0.001 |
| Calcium channel blockers, % (n) | 15.7% (80) | 2.8% (1) | 0.033 |
| Inotropic agents, % (n) | 2.6% (13) | 22.2% (8) | <0.001 |
| Levosimendan, % (n) | 0.4% (2) | 8.3% (3) | <0.001 |
| Coronary angiography, % (n) | 62.2% (316) | 19.4% (7) | <0.001 |
| LM without significant stenosis, % (n) | 45.7% (232) | 8.3% (3) | 0.002 |
| PCI, % (n) | 36.8% (187) | 5.6% (2) | <0.001 |
| Invasive MV, % (n) | 1.2% (6) | 11.1% (4) | <0.001 |
| Non-invasive MV, % (n) | 2.4% (12) | 27.7% (10) | <0.001 |
| Temporary pacemaker, % (n) | 0.8% (4) | 7.7% (3) | <0.001 |
| Complications during hospitalization | |||
| LV ejection fraction <30%, % (n) | 3.1% (16) | 30.6% (11) | <0.001 |
| HF, % (n) | 26.6% (135) | 75% (27) | <0.001 |
| Mechanical complications, % (n) | 0% | 11.1% (4) | <0.001 |
| Reinfarction, % (n) | 1.4% (7) | 7.7% (3) | 0.003 |
| Sustained VT, % (n) | 0.6% (3) | 13.9% (5) | <0.001 |
| Resuscitated CA, % (n) | 1.2% (6) | 11.1% (4) | <0.001 |
| MB, % (n) | 2.6% (13) | 8.3% (3) | <0.001 |
ACE: angiotensin-converting enzyme; ARBs: angiotensin receptor blockers; CA: cardiac arrest; HF: heart failure; IHM: in-hospital mortality; LM: left main; LV: left ventricular; MB: major bleeding; MV: mechanical ventilation; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; VT: ventricular tachycardia.
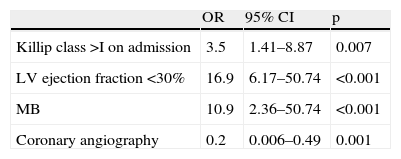
In multivariate analysis, independent predictors of IHM were Killip class >1 on admission, LV ejection fraction <30%, and MB (which increased the risk of IHM almost 11-fold). Use of coronary angiography had a protective effect (Table 3).
Variables associated with in-hospital mortality on multivariate analysis.
| OR | 95% CI | p | |
| Killip class >I on admission | 3.5 | 1.41–8.87 | 0.007 |
| LV ejection fraction <30% | 16.9 | 6.17–50.74 | <0.001 |
| MB | 10.9 | 2.36–50.74 | <0.001 |
| Coronary angiography | 0.2 | 0.006–0.49 | 0.001 |
CI: confidence interval; LV: left ventricular; MB: major bleeding; OR: odds ratio.
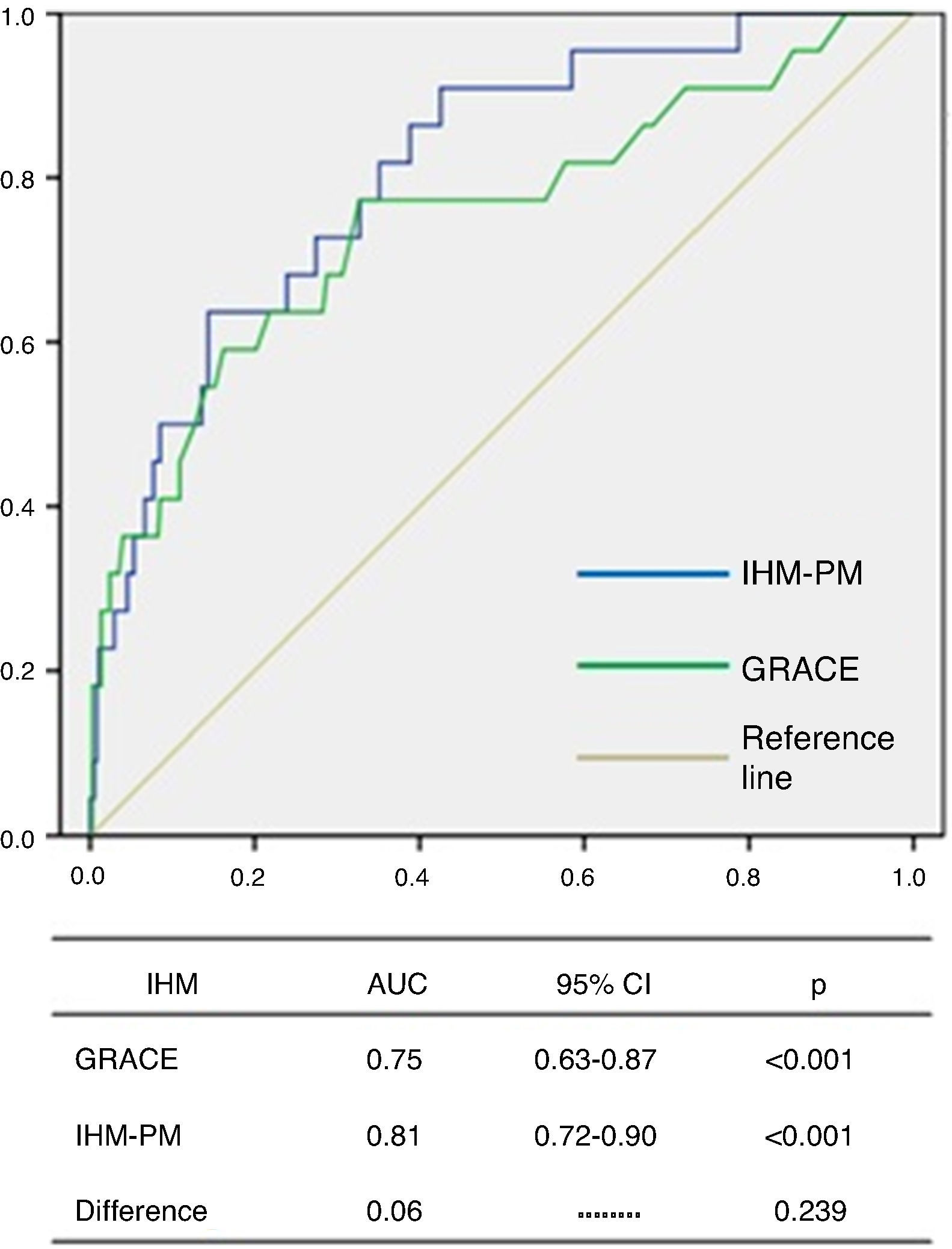
The GRACE score showed good diagnostic accuracy in identifying patients who died during hospitalization, with an area under the curve (AUC) of 0.75 (95% CI 0.63–0.87, p<0.001) (Figure 1). The Youden index for the GRACE score was 170 (sensitivity 77%, specificity 67%).
The IHM-PM presented a good fit for IHM according to the Hosmer-Lemeshow test (p=0.533). ROC curve analysis showed excellent discriminatory capacity for IHM, with an AUC of 0.81 (95% CI 0.72–0.90, p<0.001), although no significant difference was seen between the AUCs of the two models (0.06, p=0.239) (Figure 2).
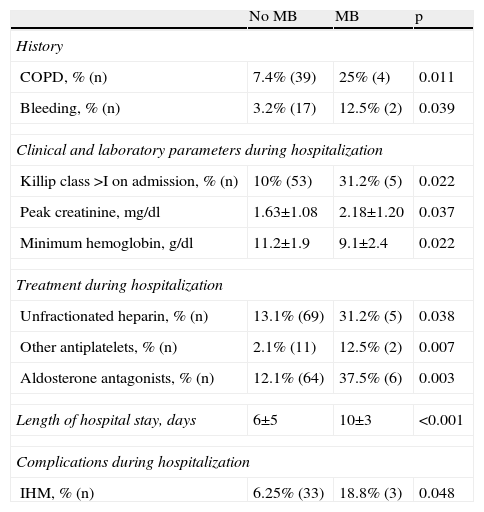
Predictors of in-hospital major bleedingMB was associated with a history of bleeding or COPD, Killip class >I on admission, higher peak creatinine and lower minimum hemoglobin, treatment with unfractionated heparin, antiplatelets other than aspirin or clopidogrel, and aldosterone antagonists, longer hospital stay and higher IHM (Table 4). On univariate analysis no association was seen between MB and age, gender, other cardiovascular risk factors, previous medication, BMI, admission hemoglobin, creatinine or platelets, medication with glycoprotein IIb/IIIa inhibitors, or coronary angiography, PCI, CABG, type of stent, or vascular access or closure devices.
Variables associated with major bleeding on univariate analysis.
| No MB | MB | p | |
| History | |||
| COPD, % (n) | 7.4% (39) | 25% (4) | 0.011 |
| Bleeding, % (n) | 3.2% (17) | 12.5% (2) | 0.039 |
| Clinical and laboratory parameters during hospitalization | |||
| Killip class >I on admission, % (n) | 10% (53) | 31.2% (5) | 0.022 |
| Peak creatinine, mg/dl | 1.63±1.08 | 2.18±1.20 | 0.037 |
| Minimum hemoglobin, g/dl | 11.2±1.9 | 9.1±2.4 | 0.022 |
| Treatment during hospitalization | |||
| Unfractionated heparin, % (n) | 13.1% (69) | 31.2% (5) | 0.038 |
| Other antiplatelets, % (n) | 2.1% (11) | 12.5% (2) | 0.007 |
| Aldosterone antagonists, % (n) | 12.1% (64) | 37.5% (6) | 0.003 |
| Length of hospital stay, days | 6±5 | 10±3 | <0.001 |
| Complications during hospitalization | |||
| IHM, % (n) | 6.25% (33) | 18.8% (3) | 0.048 |
COPD: chronic obstructive pulmonary disease; MB: major bleeding; Other antiplatelets: antiplatelets other than aspirin and clopidogrel.
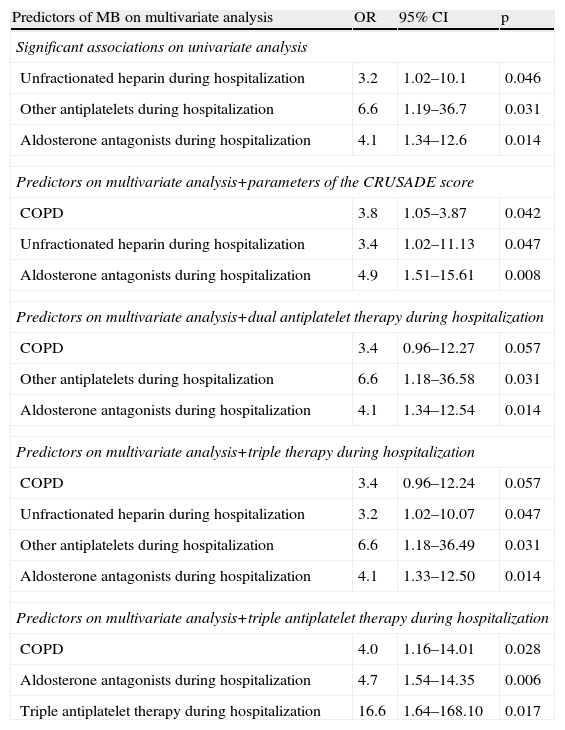
On multivariate analysis, of all the associations with statistical significance on univariate analysis, only medical therapy during hospitalization (unfractionated heparin, antiplatelets other than aspirin or clopidogrel, and aldosterone antagonists) were independent predictors of MB. The same analysis including these predictors and the parameters of the CRUSADE score identified history of COPD and medication with unfractionated heparin and aldosterone antagonists as predictors of MB. In multivariate analysis including the same predictors and dual antiplatelet or triple therapy separately, marginally significant p values were obtained for COPD and unfractionated heparin, while antiplatelets other than aspirin or clopidogrel and aldosterone antagonists were predictors of MB. In a similar analysis including triple antiplatelet therapy, this was shown to be an independent predictor of MB, as were COPD and medication with aldosterone antagonists (Table 5).
Variables associated with major bleeding on multivariate analysis.
| Predictors of MB on multivariate analysis | OR | 95% CI | p |
| Significant associations on univariate analysis | |||
| Unfractionated heparin during hospitalization | 3.2 | 1.02–10.1 | 0.046 |
| Other antiplatelets during hospitalization | 6.6 | 1.19–36.7 | 0.031 |
| Aldosterone antagonists during hospitalization | 4.1 | 1.34–12.6 | 0.014 |
| Predictors on multivariate analysis+parameters of the CRUSADE score | |||
| COPD | 3.8 | 1.05–3.87 | 0.042 |
| Unfractionated heparin during hospitalization | 3.4 | 1.02–11.13 | 0.047 |
| Aldosterone antagonists during hospitalization | 4.9 | 1.51–15.61 | 0.008 |
| Predictors on multivariate analysis+dual antiplatelet therapy during hospitalization | |||
| COPD | 3.4 | 0.96–12.27 | 0.057 |
| Other antiplatelets during hospitalization | 6.6 | 1.18–36.58 | 0.031 |
| Aldosterone antagonists during hospitalization | 4.1 | 1.34–12.54 | 0.014 |
| Predictors on multivariate analysis+triple therapy during hospitalization | |||
| COPD | 3.4 | 0.96–12.24 | 0.057 |
| Unfractionated heparin during hospitalization | 3.2 | 1.02–10.07 | 0.047 |
| Other antiplatelets during hospitalization | 6.6 | 1.18–36.49 | 0.031 |
| Aldosterone antagonists during hospitalization | 4.1 | 1.33–12.50 | 0.014 |
| Predictors on multivariate analysis+triple antiplatelet therapy during hospitalization | |||
| COPD | 4.0 | 1.16–14.01 | 0.028 |
| Aldosterone antagonists during hospitalization | 4.7 | 1.54–14.35 | 0.006 |
| Triple antiplatelet therapy during hospitalization | 16.6 | 1.64–168.10 | 0.017 |
CI: confidence interval; COPD: chronic obstructive pulmonary disease; MB: major bleeding; OR: odds ratio; Other antiplatelets: antiplatelets other than aspirin and clopidogrel.
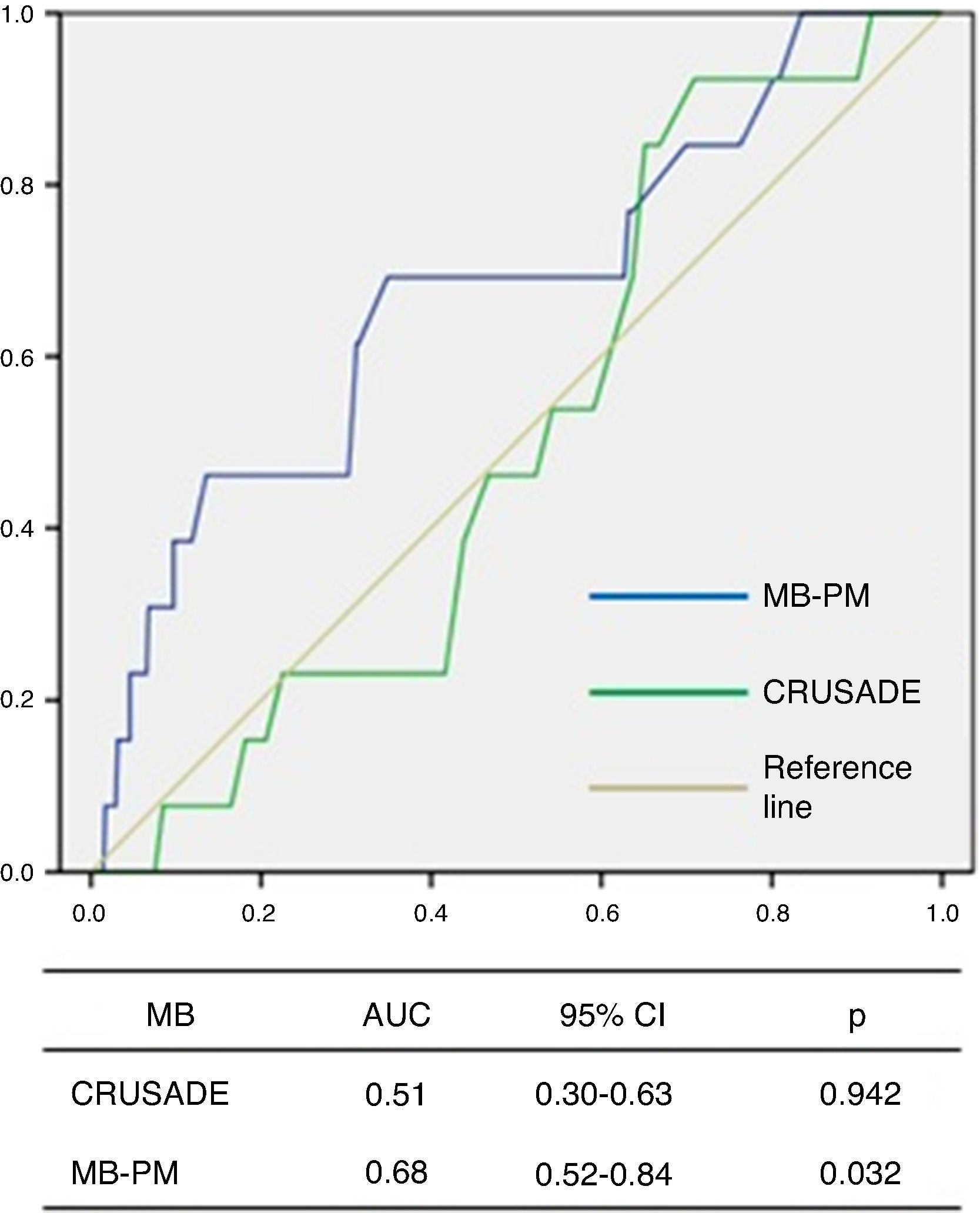
ROC curve analysis showed that the CRUSADE score had poor diagnostic accuracy in predicting MB in this population, with an AUC of 0.51 (95% CI 0.30–0.63, p=0.942; Figure 3).
The MB-PM presented a good fit on the Hosmer-Lemeshow test (p=0.111) and on ROC curve analysis showed good discriminatory capacity for MB in the study population (AUC 0.68, 95% CI 0.52–0.84, p=0.032; Figure 3).
DiscussionThe findings of this study suggest that the GRACE score is suitable for IHM risk assessment in octogenarians with NSTE-ACS, but the CRUSADE score appears to be inadequate for assessment of bleeding risk in this age-group. To our knowledge, this is the first study to assess the applicability of the GRACE and CRUSADE scores in this population.
The comorbidities and cardiovascular risk factors identified in our study were similar to those found in other series on NSTE-ACS in elderly patients.7,9
The frequency of antithrombotic therapy in our sample was similar to that in a substudy of the CRUSADE initiative in NSTE-ACS patients aged 75–89 years, except that in the CRUSADE cohort medication with glycoprotein IIb/IIIa inhibitors was more frequent (29.2% vs. 5.9%). Coronary angiography and PCI were less frequent in the CRUSADE subgroup, although results were only presented for procedures performed within 48 hours of admission, and for a different age-group.7 In the Acute Coronary Syndromes Israeli Survey9 of NSTEMI patients aged >80 years, coronary angiography was slightly less frequent than in our population (55 vs. 59.4%), in which 91% of patients were admitted for NSTEMI. Even so, 40% of these patients did not undergo angiography and 50% were not revascularized.
Coronary angiography was associated in our study with lower IHM but not with MB. PCI was also associated with lower IHM, although not independently. These findings are in agreement with those of the substudy of the Acute Coronary Syndromes Israeli Survey, in which a reduction in one-year mortality was seen following revascularization (hazard ratio [HR] 0.5, p=0.004), and a reduction in one-year (HR 0.4, p=0.04) and 30-day mortality (HR 0.38, p=0.02) with coronary angiography within 24 hours of NSTEMI in octogenarian patients. The substudy of the CRUSADE registry7 also concluded that adherence to treatment guidelines, including catheterization within 48 hours of symptom onset, was associated with lower IHM in patients aged over 75 with NSTE-ACS.
The better predictive capacity of coronary angiography compared to PCI in this population may reflect the different revascularization strategies adopted. Furthermore, PCI was not associated with MB.
It should, however, be borne in mind that there may have been a selection bias due to the physical, cognitive and social situation of the elderly; this may have led to the selection of fitter patients for invasive treatment, which is known to have prognostic benefit.9 These findings highlight the need to adjust risk stratification scores to the specific characteristics of this age-group.
The incidence of IHM and MB in the ProACS was lower than reported in other studies on similar populations, including the substudy of the CRUSADE registry7 (the largest on NSTE-ACS in the elderly, with 5557 patients), in which IHM was 7.8% (vs. 6.6%) and MB was observed in 13.1% (vs. 2.9%). The difference in MB may be due to the wider definition of bleeding used, as well as the inclusion of other criteria in the CRUSADE study, such as an absolute hematocrit drop of 12%, intracranial hemorrhage, retroperitoneal bleed, red blood cell transfusion in conjunction with a documented bleeding event if baseline hematocrit was <28%, or any red blood cell transfusion if baseline hematocrit was ≥28%.7
The results of our study clearly show the importance of comorbidities for short-term prognosis in patients aged ≥80 years, particularly HF as a predisposing factor for IHM and MB, and COPD for MB. Both are associated with chronic inflammatory states, leading to physical frailty and anemia. In the case of COPD, common therapies such as steroids may also contribute to MB.
Our results also confirm the close and independent relationship between MB and IHM. The increased risk of MB seen with therapies such as unfractionated heparin, antiplatelets other than aspirin or clopidogrel, triple antiplatelet therapy (glycoprotein IIb/IIIa inhibitors associated with dual antiplatelet therapy) and aldosterone antagonists may be due to overdosing, which is a common problem in the elderly, related to deteriorating renal function. Overdosing has also been reported with drugs that require the dose to be adjusted for body weight, such as unfractionated heparin, due to changes in body composition and protein levels in the elderly.11 In the case of antithrombotics, overdosing can increase the risk of bleeding. The association of MB with aldosterone antagonists may be due to various factors, including worsening of renal function by the drug's action and its wider use in patients with HF.
The variables in each of the models created were selected with the aim of the models being applied at hospital admission. For this reason, creatinine on admission was included in the MB-PM even though peak creatinine was of statistical significance, given the low number of variables that would have been included following the initial criteria. Hemoglobin on admission was not included since the demonstrated association of its minimum value with MB is merely a consequence of the latter.
According to our analysis, the GRACE score appropriately identified IHM in this population, without significant differences from the adjusted IHM-PM; its Youden index was higher than that of the high-risk cutoff for IHM recommended in the ESC guidelines for NSTE-ACS,1,2 which would correspond to an adjustment for this age-group. However, the CRUSADE score, which is also recommended in the guidelines, was inadequate for predicting MB in this population. This may be partly explained by the use of a different definition of MB from that in the CRUSADE study, but even so, the CRUSADE score would be expected to show some discriminatory capacity for MB, like the GUSTO criteria, but this was not the case. In an analysis of the ProACS presented at the 2012 ESC Congress,22 the CRUSADE score showed considerably better discriminatory capacity than seen in our study (AUC 0.755, 95% CI 0.69–0.82, p<0.001); however, the mean age of the population analyzed was 66±13 years.
These results demonstrate the need for new scores to predict bleeding risk in octogenarians. The MB-PM created for this study could be an alternative, since it demonstrated good diagnostic accuracy for MB. However, since the model was adjusted for this population, it would have to be validated in other samples to ensure that these results can be extended to the general population of octogenarians with NSTE-ACS.
Study limitationsOur study presents real-world data on a significant number of patients for the age-group and disease over a large geographical area. Nevertheless, certain limitations should be borne in mind when interpreting the results.
Since this was an observational study, there may have been selection bias affecting the therapies used, due to the patients’ physical, psychological, cognitive and social situation, which also affects prognosis. Another limitation is the lack of information on patients’ frailty. The ProACS contains information on comorbidities potentially associated with greater frailty, such as dementia, history of stroke, cancer, renal failure and COPD, but this association varies according to the severity of the disease. Thus, as in other studies on this subject, the different aspects of frailty are not clearly defined or distinguished (physical, cognitive, psychological or social), together with functional limitations and quality of life, even though these have been described as having a greater impact on prognosis in the elderly than the classic cardiovascular risk factors or comorbidities.11 This information would be relevant to the analysis of therapies used, MB, and IHM.
Although this was a multicenter study, the sample was selected on the basis of the inclusion criteria of the ProACS (a clinical picture suggestive of ACS within 48 hours of symptom onset) and thus may have excluded elderly patients with atypical presentation, which is common in this age-group and can delay diagnosis. Similarly, only octogenarians with NSTE-ACS who survived to hospital admission were included, and so the most severe cases were not included in the analysis. Furthermore, the fact that only 14 Portuguese centers are participating in the ProACS means that its results are not necessarily representative of the general population of octogenarians with NSTE-ACS in Portugal.
Only IHM was analyzed in this study, but long-term mortality and quality of life following discharge are also important when assessing the risks and benefits of different therapies. IHM was used as an endpoint to assess ischemic risk, but as this study shows, it also reflects bleeding risk. Ischemic risk might be more accurately assessed using an endpoint such as fatal or non-fatal MI, but this would entail long-term follow-up, which would further reduce the sample size.
ConclusionsIn patients aged ≥80 years with NSTE-ACS, the GRACE score was suitable for predicting IHM, but the CRUSADE score was inadequate for assessment of bleeding risk in this age-group. Signs of heart failure on admission, LV ejection fraction <30% and MB were independent predictors of IHM. The main predictors of MB were medication during hospitalization and history of COPD. Use of coronary angiography was associated with better prognosis in terms of IHM and did not increase the risk of MB. These findings suggest that an invasive strategy is beneficial in selected patients, and that new scores are required to assess bleeding risk in this age-group.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank all the ProACS investigators who took part in the collection of the data presented in this study.
Please cite this article as: Faustino A, Mota P, Silva J, Em nome dos Investigadores do Registo Nacional de Síndromes Coronárias Agudas da Sociedade Portuguesa de Cardiologia. Síndromes coronárias agudas sem supradesnivelamento-ST nos octogenários: aplicabilidade dos scores GRACE e CRUSADE. Rev Port Cardiol. 2014;33:617–627.


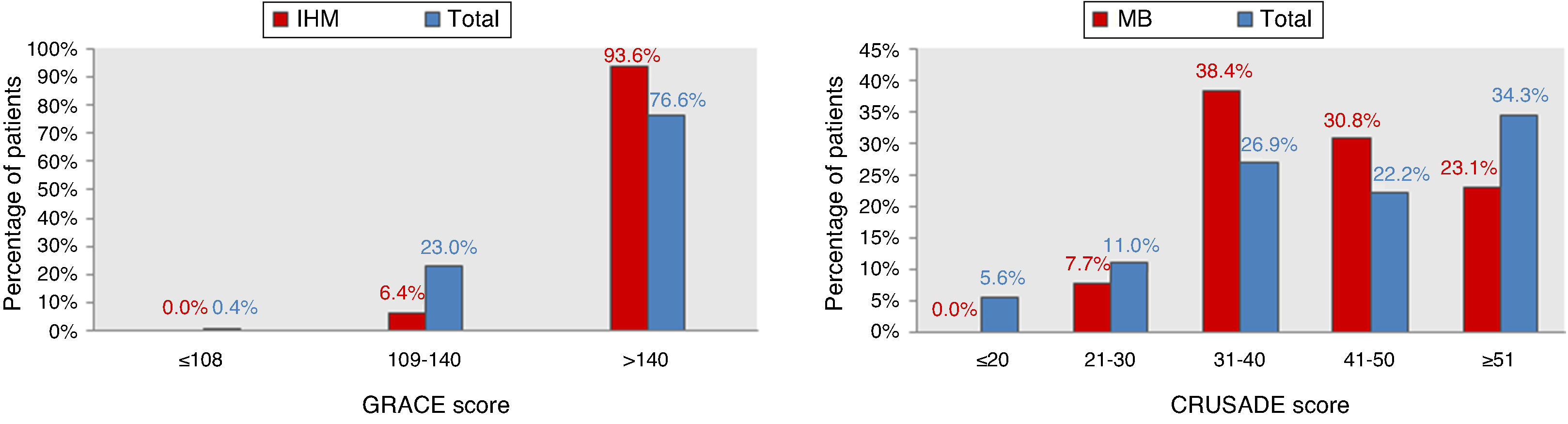
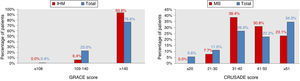 IHM: in-hospital mortality; MB: major bleeding.' title='Distribution of the total population and of patients with events according to GRACE and CRUSADE scores.
IHM: in-hospital mortality; MB: major bleeding.' title='Distribution of the total population and of patients with events according to GRACE and CRUSADE scores. 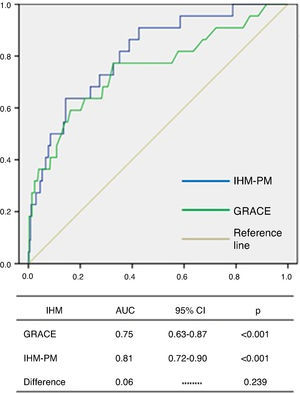 IHM-PM for assessment of in-hospital mortality.
IHM-PM for assessment of in-hospital mortality. 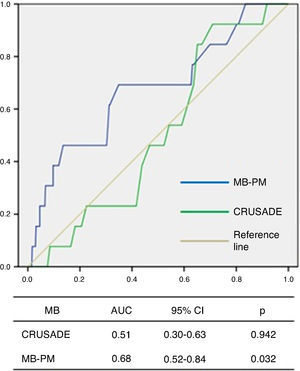 AUC: area under the curve; CI: confidence interval; MB-PM: major bleeding prediction model.' title='ROC curve analysis of the CRUSADE score and MB-PM for assessment of major bleeding.
AUC: area under the curve; CI: confidence interval; MB-PM: major bleeding prediction model.' title='ROC curve analysis of the CRUSADE score and MB-PM for assessment of major bleeding. 
