To assess the prevalence of musculoskeletal complaints and their association with risk factor profile and functional and psychosocial status in patients on a cardiac rehabilitation program.
MethodsIn this cross-sectional study of 449 patients admitted within three months of an acute coronary syndrome, patients were divided into those with (MSC+) and those without (MSC−) musculoskeletal complaints. The Hospital Anxiety and Depression Scale and the Short Form 36 Health Survey were used to assess psychosocial status and quality of life, and the International Physical Activity Questionnaire for physical activity. Functional capacity was estimated from exercise testing.
ResultsMusculoskeletal pain was present in 119 patients (27%), mainly in the lower limbs (56%). MSC+ were older (mean 56.5±9.9 vs. 53.2±9.5 years; p<0.001) and more frequently women (20.2% vs. 9.1%; p<0.001). MSC+ had a higher prevalence of dyslipidemia (68.6% vs. 51.2%; p<0.001), hypertension (51.7% vs. 35.5%; p<0.001), obesity (29.4% vs. 17.9%; p<0.001) and metabolic syndrome (44.5% vs. 31.5%; p<0.001). MSC+ showed higher body mass index and waist circumference, and lower physical activity levels (p<0.05), as well as lower functional capacity (8.6±2.2 vs. 9.6±2.1 MET; p<0.05), higher scores for depression (6 [3–9] vs. 3 [1–7]; p<0.05) and anxiety (7 [3–10] vs. 5 [2–8]; p<0.05), and lower scores for physical (44.1±8.7 vs. 47.6±7.6; p<0.05) and mental (39.2±13.0 vs. 44.0±13.0; p<0.05) quality of life.
ConclusionsMusculoskeletal complaints are common in cardiac rehabilitation and predict lower levels of physical activity, worse cardiovascular risk factor profile, and poorer functional capacity and psychosocial status, irrespective of age and gender.
determinar a prevalência da dor músculo-esquelética e sua associação com perfil de risco cardiovascular, estado funcional e psicossocial em doentes admitidos num programa de reabilitação cardíaca
Métodosestudo transversal de 449 doentes admitidos nos primeiros três meses após síndroma coronária aguda. Os doentes foram categorizados em grupo com (MSC+) e sem (MSC-) dor músculo-esquelética. O estado psicossocial e a qualidade foram avaliadas com as escalas HADS e SF36 e a atividade física com o IPAQ. Capacidade funcional foi estimada com prova de esforço basal.
ResultadosDor músculo-esquelética estava presente em 119 (27%), maioritariamente nos membros inferiores (56%). MSC+ eram mais velhos [média(DP): 56,5±9,9 versus 53,2±9,5; p<0.001] e mais frequentemente mulheres [20,2% versus 9,1%; p<0,001]. MSC+ apresentavam maior prevalência de dislipidemia (68,6% versus 51,2%; p<0,001), hipertensão arterial (51,7% versus 35,5%; p<0,001), obesidade (29,4% versus 17,9%; p<0,001) e síndroma metabólica (44,5% versus 31,5%; p<0,001). MSC+ mostravam maiores índice de massa corporal e perímetro abdominal e menor atividade física (p<0,005). MSC+ tinham menor capacidade funcional [8,6±2,2 versus 9,6±2,1 MET; p<0,05], maior prevalência de sintomas depressivos [6(3-9) versus 3(1-7); p<0,05] e ansiosos [7(3-10) versus 5(2-8); p<0,05], e menor qualidade de vida física [44,1±8,7 versus 47,6±7,6; p<0,05] e mental [39,2±13,0 versus 44,0±13,0; p<0,05].
ConclusãoDor músculo-esquelética associa-se a menores níveis de atividade física, pior capacidade funcional, pior perfil de risco cardiovascular e a um estado psicossocial mais adverso.
Despite recent advances in both revascularization procedures and drug therapy, coronary heart disease (CHD) remains the major cause of death and disability worldwide, and is expected to remain so for at least the next 20 years.1,2 Population aging, together with a sharp decrease in case fatality, have resulted in a growing population of CHD survivors who, over time, frequently develop significant disability, restrictions in work and social participation, and need for continuous medical assistance.3
The prevalence of musculoskeletal complaints (MSC) also increases with age and is a major cause of disability and functional impairment.1 It has been shown that CHD patients have double the risk of suffering from arthritis and 30% higher probability of being physically inactive compared to the general population, even adjusting for age, gender, race/ethnicity, education level, and body mass index (BMI).4 However, several mechanisms may contribute to this association, particularly restricted physical activity,4 unhealthy lifestyles, low-grade inflammatory activation,5 and prolonged use of anti-inflammatory drugs.6
Cardiac rehabilitation (CR) offers an excellent opportunity for identification and early management of MSC, helping to overcome barriers that restrict activity and providing individualized exercise programs.
Our objectives were: (1) to assess the prevalence of MSC in patients undergoing CR; (2) to study the association between MSC and cardiovascular (CV) risk profile and functional and psychosocial status; and (3) to estimate the increased prevalence of different CV risk factors due to the presence of MSC.
MethodsStudy designThis was a single-center hospital-based cross-sectional study. The CR program took place in a cardiac rehabilitation unit. Enrollment in CR was voluntary after referral from the attending cardiologist during the initial hospitalization. Written informed consent was obtained from all patients at program enrollment.
ParticipantsPatients were considered eligible for CR if, in the three months before referral, they had one of the following: acute coronary syndrome, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Exclusion criteria followed the standard recommendations for phase II CR programs7: patients were excluded if they were considered unable to safely engage in exercise sessions (due to neuromuscular, orthopedic or cognitive impairment), had uncontrolled diabetes or unfavorable blood pressure response to exercise, ejection fraction <30%, New York Heart Association functional class ≥3, complex ventricular or supraventricular arrhythmias, or high-grade atrioventricular block.
Of a total of 487 patients initially approached for CR participation, 38 (7.8%) refused to participate due to various constraints (accessibility 22; resumption of work 10; financial issues 6). A secondary analysis showed that, compared to those undergoing CR, those who did not attend the CR sessions were more frequently women (9 [16.7%] vs. 54 [12.0%], p=0.03) and were more likely to present previous CHD (37.8% vs. 16.8; p<0.05), hypertension (52.6% vs. 39.7%, p=0.05), and active smoking (71.4% vs. 55.7%; p<0.05). No between-group differences were detected in age, BMI, waist circumference, or prevalence of diabetes, dyslipidemia, or metabolic syndrome.
Outcome measuresSociodemographic and clinical characteristicsSociodemographic characteristics were assessed through a structured questionnaire and included marital status, years of education, and work status. Information regarding the index event, revascularization procedure, CV risk factors, cardiac medications, and comorbidities were obtained from medical records.
Anthropometric parameters (weight, height, BMI and waist circumference) were measured at baseline in accordance with the recommendations of the US National Institutes of Health.8
Physical activity and functional capacityLeisure time physical activity was assessed using the International Physical Activity Questionnaire, measured in metabolic equivalents (MET)-min/week. Only non-professional physical activities and those lasting at least 15 min were included in the analysis.9 We used a cut-off of 600 MET-min/week (30 min/day, 7 days/week) of moderate intensity physical activity.10
Functional capacity was estimated from baseline symptom-limited maximal exercise using the standard Bruce protocol, and quantified by the amount of work performed in multiples of resting oxygen uptake (defined as 1 MET=3.5 ml oxygen per kg body weight per min). Standard tables were used to convert treadmill grade and speed into estimated MET levels.11
Musculoskeletal complaintsMSC were characterized using an interviewer-administered Portuguese version of the Nordic Musculoskeletal Questionnaire.12,13 This consists of 40 questions regarding location (nine body areas: neck, shoulders, elbows, wrist/hands, mid and low back, hips/thighs, knee, ankles/feet), duration (last 12 months; last 7 days) and pain intensity (numeric scale). Since we aimed to establish the influence of long-standing MSC on CV risk factors and functional and psychosocial status, all patients reporting pain in the last 12 months were included in the analysis.
Psychosocial factorsThe Hospital Anxiety and Depression Scale was used to assess the presence and severity of anxiety and depressive symptoms. It has been validated and used extensively in CHD research.14 Each domain consists of seven questions, scored between 0 (no symptoms) and 3 (severe symptoms), with a total score ranging between 0 and 21. A cut-off of 8 in either domain was used to define clinical anxiety and depression.15
The Short Form 36 Health Survey (SF-36, version 2), which is widely used and validated for CHD populations, was used to measure both physical and mental dimensions of quality of life. It consists of eight categories, four in each domain (physical and mental), to provide physical and mental health summary scores.16,17 Scores in each category range between 0 (worst) and 100 (best) quality of life. Scores are presented after standardization through linear transformations to achieve a mean score of 50 and standard deviation of 10.18
Statistical analysisThe patients were divided into those with (MSC+) and those without (MSC−) musculoskeletal complaints. Between-group comparisons were made using the standard t test and the Mann-Whitney test for normal and non-normal continuous variables, respectively. Categorical variables were compared using the chi-square test. Binomial logistic regression was used to assess the prevalence of categorical CV risk factors through categories of MSC, with adjustment for age and gender.
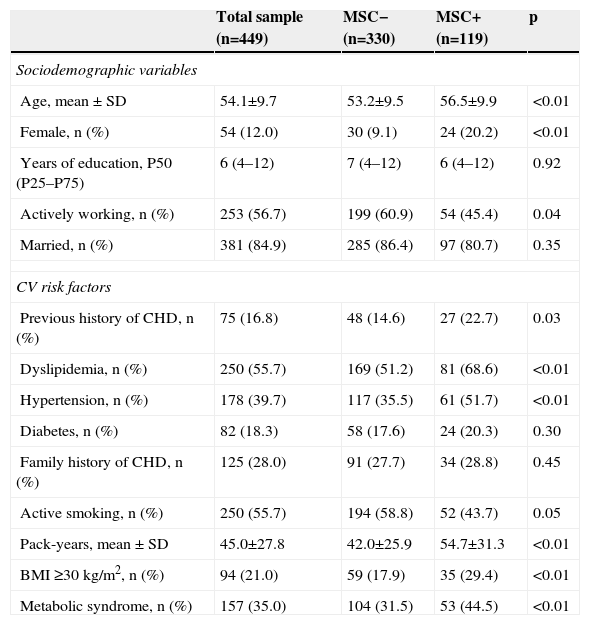
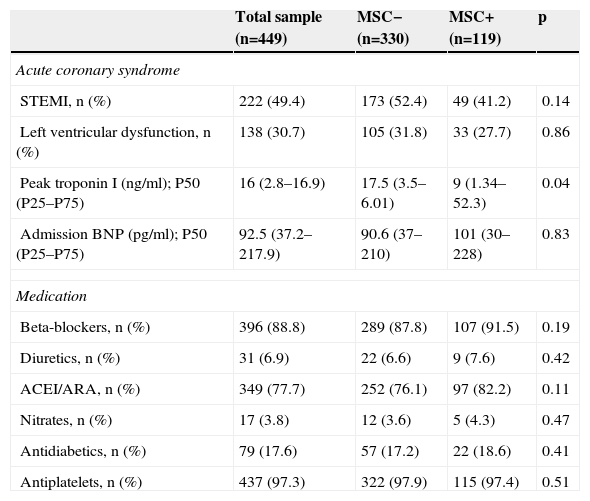
ResultsThe study sample consisted of 449 patients, mostly male (88.0%), with low educational level (<9 years of education: 44.0%), married (85.0%), and actively working (57.0%) (Table 1). Almost half presented with ST-segment elevation myocardial infarction (49.0%), with 83.0% undergoing PCI, 49.0% CABG (9.4%), and 7.0% receiving only medical treatment (Table 2). There were no differences between groups regarding cardioprotective drug use (Table 2).
Sociodemographic characteristics and cardiovascular risk factors in the different groups and the total sample.
| Total sample (n=449) | MSC− (n=330) | MSC+ (n=119) | p | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, mean ± SD | 54.1±9.7 | 53.2±9.5 | 56.5±9.9 | <0.01 |
| Female, n (%) | 54 (12.0) | 30 (9.1) | 24 (20.2) | <0.01 |
| Years of education, P50 (P25–P75) | 6 (4–12) | 7 (4–12) | 6 (4–12) | 0.92 |
| Actively working, n (%) | 253 (56.7) | 199 (60.9) | 54 (45.4) | 0.04 |
| Married, n (%) | 381 (84.9) | 285 (86.4) | 97 (80.7) | 0.35 |
| CV risk factors | ||||
| Previous history of CHD, n (%) | 75 (16.8) | 48 (14.6) | 27 (22.7) | 0.03 |
| Dyslipidemia, n (%) | 250 (55.7) | 169 (51.2) | 81 (68.6) | <0.01 |
| Hypertension, n (%) | 178 (39.7) | 117 (35.5) | 61 (51.7) | <0.01 |
| Diabetes, n (%) | 82 (18.3) | 58 (17.6) | 24 (20.3) | 0.30 |
| Family history of CHD, n (%) | 125 (28.0) | 91 (27.7) | 34 (28.8) | 0.45 |
| Active smoking, n (%) | 250 (55.7) | 194 (58.8) | 52 (43.7) | 0.05 |
| Pack-years, mean ± SD | 45.0±27.8 | 42.0±25.9 | 54.7±31.3 | <0.01 |
| BMI ≥30 kg/m2, n (%) | 94 (21.0) | 59 (17.9) | 35 (29.4) | <0.01 |
| Metabolic syndrome, n (%) | 157 (35.0) | 104 (31.5) | 53 (44.5) | <0.01 |
BMI: body mass index; CHD: coronary heart disease; CV: cardiovascular; MSC−: patients without musculoskeletal complaints; MSC+: patients with musculoskeletal complaints; P50 (P25–P75): median (interquartile range); SD: standard deviation.
Acute coronary event characteristics and medication use in the different groups and the total sample.
| Total sample (n=449) | MSC− (n=330) | MSC+ (n=119) | p | |
|---|---|---|---|---|
| Acute coronary syndrome | ||||
| STEMI, n (%) | 222 (49.4) | 173 (52.4) | 49 (41.2) | 0.14 |
| Left ventricular dysfunction, n (%) | 138 (30.7) | 105 (31.8) | 33 (27.7) | 0.86 |
| Peak troponin I (ng/ml); P50 (P25–P75) | 16 (2.8–16.9) | 17.5 (3.5–6.01) | 9 (1.34–52.3) | 0.04 |
| Admission BNP (pg/ml); P50 (P25–P75) | 92.5 (37.2–217.9) | 90.6 (37–210) | 101 (30–228) | 0.83 |
| Medication | ||||
| Beta-blockers, n (%) | 396 (88.8) | 289 (87.8) | 107 (91.5) | 0.19 |
| Diuretics, n (%) | 31 (6.9) | 22 (6.6) | 9 (7.6) | 0.42 |
| ACEI/ARA, n (%) | 349 (77.7) | 252 (76.1) | 97 (82.2) | 0.11 |
| Nitrates, n (%) | 17 (3.8) | 12 (3.6) | 5 (4.3) | 0.47 |
| Antidiabetics, n (%) | 79 (17.6) | 57 (17.2) | 22 (18.6) | 0.41 |
| Antiplatelets, n (%) | 437 (97.3) | 322 (97.9) | 115 (97.4) | 0.51 |
ACEI: angiotensin-converting enzyme inhibitors; ARA: aldosterone receptor antagonists; BNP: brain natriuretic peptide; MSC−: patients without musculoskeletal complaints; MSC+: patients with musculoskeletal complaints; P50 (P25–P75): median (interquartile range); STEMI: ST-segment elevation myocardial infarction.
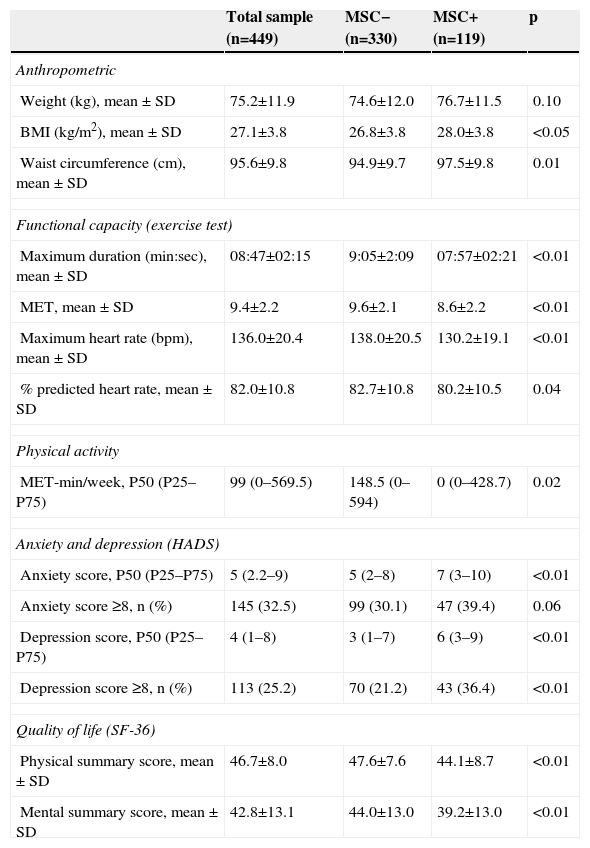
Of the study sample, 119 (26.5%) patients had MSC, 68 (58.0%) in the lower limbs, 40 (34.0%) in the spine and nine (7.7%) in the upper limbs. Among those with lower limb complaints, the majority reported knee pain (46; 68.7%) or ankle/foot pain (14; 20.3%). Mean reported visual analog scale pain score was 4.2±1.2 (range 1–9). Patients reporting MSC were older, more frequently women, and had a worse CV risk factor profile, with higher prevalences of dyslipidemia, hypertension, overweight, and metabolic syndrome. Despite a higher proportion of active smokers in the MSC− group, pack-year history was higher in MSC+, probably reflecting older age in the latter. No differences were found regarding diabetes or family history of CHD (Table 1). The MSC+ group showed higher BMI and more abdominal obesity (Table 3).
Anthropometric characteristics, functional capacity, physical activity and psychosocial status in the different groups and the total sample.
| Total sample (n=449) | MSC− (n=330) | MSC+ (n=119) | p | |
|---|---|---|---|---|
| Anthropometric | ||||
| Weight (kg), mean ± SD | 75.2±11.9 | 74.6±12.0 | 76.7±11.5 | 0.10 |
| BMI (kg/m2), mean ± SD | 27.1±3.8 | 26.8±3.8 | 28.0±3.8 | <0.05 |
| Waist circumference (cm), mean ± SD | 95.6±9.8 | 94.9±9.7 | 97.5±9.8 | 0.01 |
| Functional capacity (exercise test) | ||||
| Maximum duration (min:sec), mean ± SD | 08:47±02:15 | 9:05±2:09 | 07:57±02:21 | <0.01 |
| MET, mean ± SD | 9.4±2.2 | 9.6±2.1 | 8.6±2.2 | <0.01 |
| Maximum heart rate (bpm), mean ± SD | 136.0±20.4 | 138.0±20.5 | 130.2±19.1 | <0.01 |
| % predicted heart rate, mean ± SD | 82.0±10.8 | 82.7±10.8 | 80.2±10.5 | 0.04 |
| Physical activity | ||||
| MET-min/week, P50 (P25–P75) | 99 (0–569.5) | 148.5 (0–594) | 0 (0–428.7) | 0.02 |
| Anxiety and depression (HADS) | ||||
| Anxiety score, P50 (P25–P75) | 5 (2.2–9) | 5 (2–8) | 7 (3–10) | <0.01 |
| Anxiety score ≥8, n (%) | 145 (32.5) | 99 (30.1) | 47 (39.4) | 0.06 |
| Depression score, P50 (P25–P75) | 4 (1–8) | 3 (1–7) | 6 (3–9) | <0.01 |
| Depression score ≥8, n (%) | 113 (25.2) | 70 (21.2) | 43 (36.4) | <0.01 |
| Quality of life (SF-36) | ||||
| Physical summary score, mean ± SD | 46.7±8.0 | 47.6±7.6 | 44.1±8.7 | <0.01 |
| Mental summary score, mean ± SD | 42.8±13.1 | 44.0±13.0 | 39.2±13.0 | <0.01 |
BMI: body mass index; HADS: Hospital Anxiety and Depression Scale; MET: metabolic equivalents estimated from the IPAQ; P50 (P25–P75): median (interquartile range); P50 (P25–P75): median (interquartile range); SD: standard deviation, SF-36: Short Form 36 Health Survey.
MSC+ patients were less active, with 65% reporting less than 600 MET-min/week, compared to 44% in the MSC− group (p<0.05). Functional capacity was, on average, 10% lower in MSC+ compared to MSC− (Table 3).
Psychosocial status and quality of lifeSymptoms of depression and anxiety were more prevalent and of greater severity in MSC+, with 36.0% MSC+ and 21.0% MSC− patients presenting clinical depression (p<0.05), and 39.0% MSC+ and 30.0% MSC− having clinical anxiety (p=0.06) (Table 3). Both physical and mental dimensions of quality of life were significantly lower in MSC+ patients (Table 3).
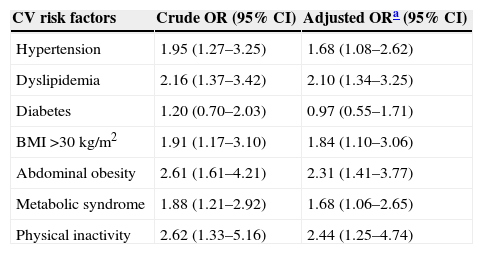
Increased risk for cardiovascular risk factors according to musculoskeletal complaint statusUnivariate logistic regression showed that MSC+ patients had double the risk of having hypertension, dyslipidemia, and obesity (both general and visceral) (Table 4). Physical inactivity was 2.4 times more prevalent in MSC+ compared to MSC− patients. Even after adjustment for age and gender, MSC status remained a strong predictor of CV risk factors, except for diabetes.
Association between musculoskeletal complaints and major cardiovascular risk factors (n=449).
| CV risk factors | Crude OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|
| Hypertension | 1.95 (1.27–3.25) | 1.68 (1.08–2.62) |
| Dyslipidemia | 2.16 (1.37–3.42) | 2.10 (1.34–3.25) |
| Diabetes | 1.20 (0.70–2.03) | 0.97 (0.55–1.71) |
| BMI >30 kg/m2 | 1.91 (1.17–3.10) | 1.84 (1.10–3.06) |
| Abdominal obesity | 2.61 (1.61–4.21) | 2.31 (1.41–3.77) |
| Metabolic syndrome | 1.88 (1.21–2.92) | 1.68 (1.06–2.65) |
| Physical inactivity | 2.62 (1.33–5.16) | 2.44 (1.25–4.74) |
BMI: body mass index; CI: confidence interval; CV: cardiovascular; OR: odds ratio.
MSC and CHD are common chronic conditions associated with significant disability and use of healthcare resources.1 In this study we showed that MSC are not only common in the CHD population but are also associated with lower levels of physical activity and a worse CV risk factor profile, estimated functional capacity, and psychosocial status, irrespective of age and gender.
The prevalence of MSC has been shown to be higher in CHD patients than in the general population. The US Centers for Disease Control estimated that the age and gender-adjusted prevalence of arthritis was 57.4% in CHD patients compared to 27.4% in the general population,4 while Marzollini et al. reported a 56.0% prevalence of MSC in a multicenter cross-sectional study of 1803 CHD patients.19 The lower prevalence found in our study (27.0%) is probably related to age-related referral bias commonly seen in CR, especially under-representation of older patients.20
Physical inactivity has been linked to increased risk for CHD in several prospective cohort studies.21,22 An aggregate analysis of prospective primary prevention studies looking at physical activity levels and CV mortality found a median risk reduction of up to 40% in more compared to less active subjects, irrespective of age, gender and race/ethnicity.23 Our results confirm the cumulative negative effect of CHD and MSC on physical activity levels, with an almost 30% increase in the prevalence of physical inactivity compared to CHD patients without MSC.4 Moreover, reduced physical activity translates into lower functional capacity, which in turn is linked to higher CV morbidity and mortality.24
Clustering of adverse CV risk factors has been described in patients with MSC.4,19,25 Our study, like others,19 confirms that patients with MSC are older, more sedentary and more frequently dyslipidemic and hypertensive compared with those without MSC. Exercise training exerts beneficial effects in both prevention and treatment of CHD and MSC. However, the impact of exercise on CV risk factor burden is controversial.26,27 Despite a clear dose-response curve between exercise and cardiometabolic risk factors, the magnitude of the effect appears modest and highly variable according to individual and exercise program characteristics.28 Methodological difficulties regarding study design, patient selection, lack of standardization of exercise and physical activity protocols, and heterogeneity of instruments for measuring physical activity (including self-reported questionnaires, logbooks, pedometers and triaxial accelerometers) hinder conclusions regarding optimal dose and type of exercise for CV prevention. Since age and gender are related to CV risk factors, CHD, and MSC, we used an age- and gender-adjusted logistic regression model to obtain a clearer picture of the influence of MSC status on prevalence of classical risk factors. The study results confirm our prior assumption of an association of MSC with a worse CV risk factor profile.
Both chronic pain due to musculoskeletal disturbances and CHD have a considerable impact on the psychosocial dimension of quality of life. The Longitudinal Aging Study Amsterdam followed 2285 individuals aged 55–85 for up to six years and found an increased risk for depression in both cardiac (+33%) and musculoskeletal (+36%) cases.29 Our study replicated both the incidence of depression in CHD and the cumulative effect of coexisting CHD and MSC.
It is therefore recommended to systematically assess psychosocial status in CR settings and to develop and implement psycho-behavioral interventions and support.
Study limitationsMSC were self-reported and no further clinical or imaging assessment was undertaken. However, any misclassification was probably non-differential and, since our purpose was to study the association between CHD and MSC status and not causation, we do not expect this to have a significant impact on the study's internal validity.
Self-reporting physical activity through questionnaires has been widely used in several observational studies, although low precision and sensitivity to change might introduce measurement biases and differential misclassifications.30 Ideally, objective measurements using biaxial or triaxial accelerometers would minimize these concerns, but cost and applicability in daily clinical practice excluded this option. On the other hand, the results and the direction associations found between physical activity, functional capacity and anthropometric characteristics indicate that the chosen instrument is measuring physical activity appropriately in this setting.
In addition to the aforementioned limitations, this was a single-center cross-sectional study of young, mostly male CHD patients, so its results cannot be extrapolated to CHD populations with different characteristics.
ConclusionsMore than a quarter of CHD patients enrolled in CR programs report MSC, which are associated with a worse CV risk factor profile, lower levels of physical activity and functional capacity, and unfavorable psycho-emotional and quality of life scores. This subgroup of CR patients is particularly susceptible to prolonged disability from CHD, productivity losses, and early retirement. Aside from indirect costs, they are also at increased risk for CHD recurrence and need for further medical assistance and revascularization procedures. Addressing MSC in a secondary prevention setting might be beneficial, coupling standard exercise training programs with activity and exercise modifications, modalities, and individualized exercise counseling.
Further research is needed to determine if and how MSC affect the health benefits of CR, and which interventions and program designs are best suited to minimize the deleterious effects of MSC on CV health.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.