Constrictive pericarditis is a clinical condition characterized by the appearance of signs and symptoms of right heart failure due to loss of pericardial compliance. Cardiac surgery is now one of the most frequent causes in developed countries, while tuberculosis remains the most prevalent cause in developing countries. Malignancy is a rare cause but usually has a poor prognosis. The diagnosis of constrictive pericarditis remains a clinical challenge and requires a combination of noninvasive diagnostic methods (echocardiography, cardiac magnetic resonance and computed tomography); in some cases, cardiac catheterization is needed to confirm the diagnosis. The authors present the case of a 51-year-old man, hospitalized due to cardiac tamponade. Diagnostic investigation was suggestive of tuberculous etiology. Despite directed medical therapy, the patient developed effusive-constrictive physiology. He underwent pericardiectomy and anatomopathologic study suggested a neoplastic etiology. The patient died in the postoperative period from biventricular failure.
A pericardite constritiva é uma entidade clínica caracterizada pelo aparecimento de sinais e sintomas de insuficiência cardíaca direita, secundários à perda da compliance pericárdica. Atualmente, a cirurgia cardíaca tornou-se numa das etiologias mais frequentes nos países desenvolvidos, mantendo-se a tuberculose como a causa mais prevalente nos países em vias de desenvolvimento. As etiologias neoplásicas são mais raras e habitualmente de pior prognóstico. O diagnóstico desta entidade mantém-se um desafio clínico, sendo necessária a integração dos achados dos métodos de diagnóstico não invasivos (ecocardiografia, ressonância magnética e tomografia computorizada) e por vezes o recurso ao cateterismo cardíaco. Os autores apresentam o caso clínico de um homem de 51 anos de idade, internado por tamponamento cardíaco. A investigação etiológica foi sugestiva de etiologia tuberculosa, que apesar da terapêutica médica dirigida, evoluiu para fisiologia efusiva-constritiva. Foi submetido a pericardiectomia e o exame anátomo-patológico sugeriu etiologia neoplásica. O doente veio a falecer no pós-operatório em falência biventricular.
Constrictive pericarditis is a clinical condition characterized by the appearance of signs and symptoms of right heart failure due to loss of pericardial compliance.1 Cardiac surgery, radiotherapy and idiopathic pericarditis are now the most common causes in developed countries, but tuberculosis remains the most prevalent cause in developing countries.1
Diagnosis is usually established by Doppler echocardiography, but doubtful cases may require other diagnostic methods, such as cardiac magnetic resonance (CMR), computed tomography (CT) or invasive hemodynamic assessment by cardiac catheterization.
Treatment is based on administration of diuretics and directed therapy for the underlying condition. However, pericardiectomy is the only definitive and potentially curative treatment and is recommended in cases with signs of chronicity.
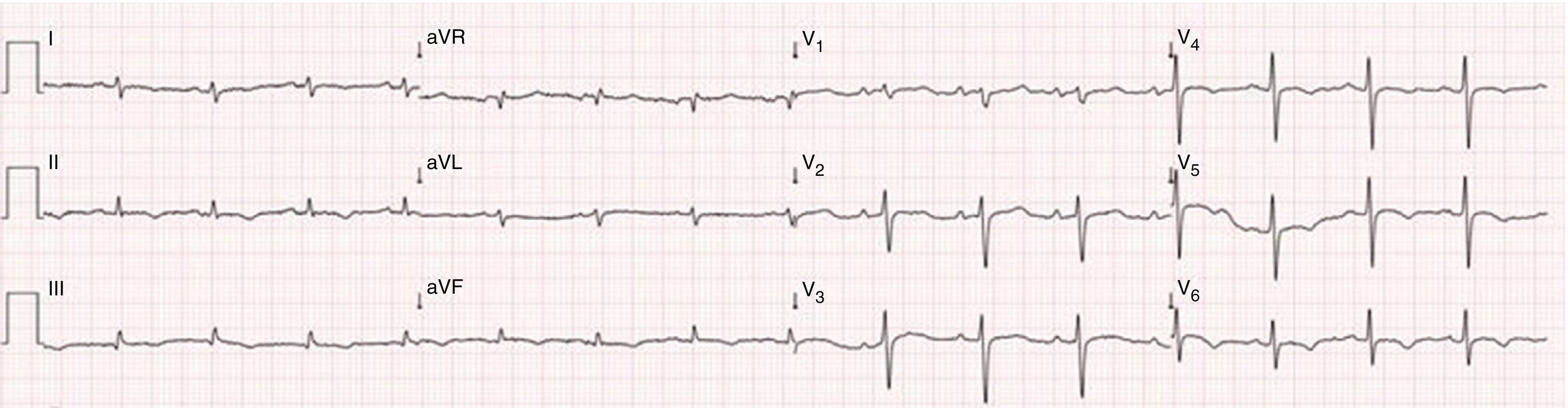
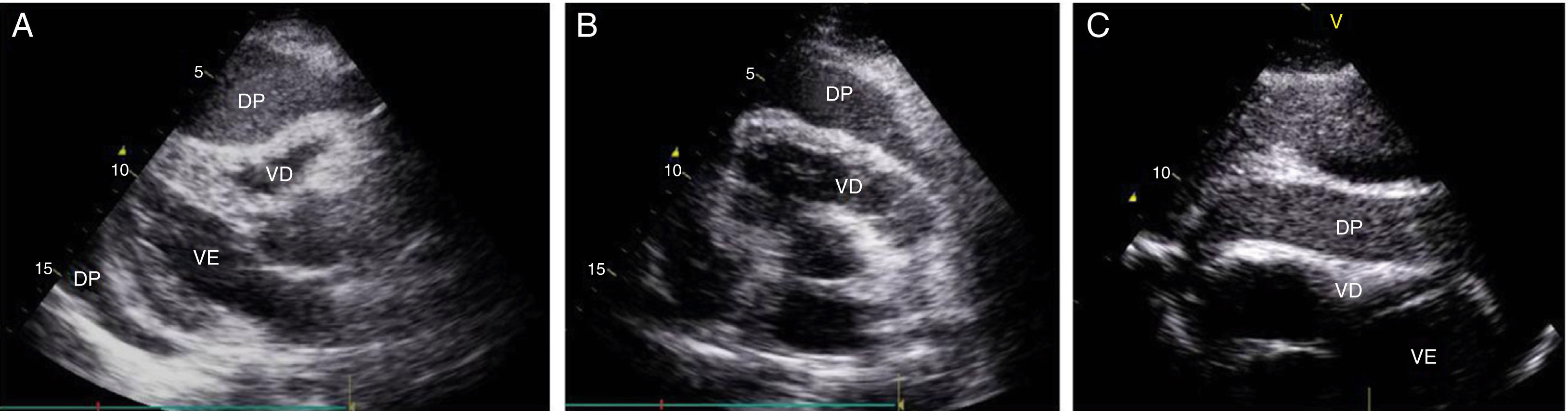
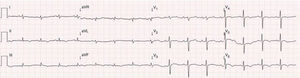
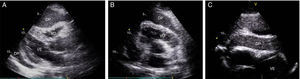
Case reportA 51-year-old man, black, regularly participating in sports and with no relevant medical history, came to the emergency department for progressively worsening fatigue, occasional sharp chest pain, increased abdominal volume, loss of appetite and food intolerance with episodes of vomiting for around a week. Three days before admission, he had an episode of syncope and noticed significant jugular distension. He denied fever, chills, cough, expectoration or weight loss. On physical examination, his blood pressure was 118/78 mmHg and heart rate was 98 bpm; he was apyretic and dyspneic, with jugular distension and faint heart sounds, but no peripheral edema. The electrocardiogram (ECG) showed sinus rhythm, with low QRS voltage in the classical and limb leads and an S1Q3T3 pattern (Figure 1). Laboratory tests showed slight elevation of inflammatory parameters (leukocytes 11.6×109/l, C-reactive protein 3.9 mg/dl), marked D-dimer elevation (5153 ng/ml), hemoglobin 13.3 g/dl, creatinine 1.3 mg/dl and negative myocardial necrosis markers. Chest CT angiography excluded pulmonary thromboembolism but revealed a large right pericardial and pleural effusion. Echocardiography showed a large, circumferential pericardial effusion, cloudy in appearance, with a maximum size of 32 mm, and partial right chamber collapse (Figure 2).
Two-dimensional transthoracic echocardiogram in (A) parasternal long-axis, (B) parasternal short-axis and (C) subcostal views, showing large circumferential pericardial effusion, cloudy in appearance, and partial right-chamber collapse. DP: pericardial effusion; VD: right ventricle; VE: left ventricle.
Pericardiocentesis was performed under fluoroscopic guidance for diagnostic and therapeutic purposes, a total of 4000 cc of bloody fluid being drained; cytochemical study revealed this to be an exudate, with normal adenosine deaminase levels. Cytology revealed no cancer cells and microbiological study, both direct and in culture, was negative, including for mycobacteria. Various markers of autoimmune disease (antinuclear antibody, rheumatoid factor, anti-cardiolipin, anti-beta-2 glycoprotein and C3 and C4 levels) and blood cancers (carcinoembryonic antigen, cancer antigen 19.9, prostate-specific antigen, alpha-fetoprotein, beta-2 microglobulin and peripheral blood smear) were analyzed, none being positive for diagnosis. Investigation of viral antibodies (echovirus, Coxsackie, adenovirus, influenza A and B, human immunodeficiency virus, and hepatitis B and C) was negative or not suggestive of recent infection. Chest-abdominal-pelvic CT revealed polyserositis only, with no adenopathy or other suspicious masses. A subsequent interferon-gamma release assay was positive, and so a diagnosis of tuberculous pericarditis was assumed and therapy was begun with antituberculosis drugs.
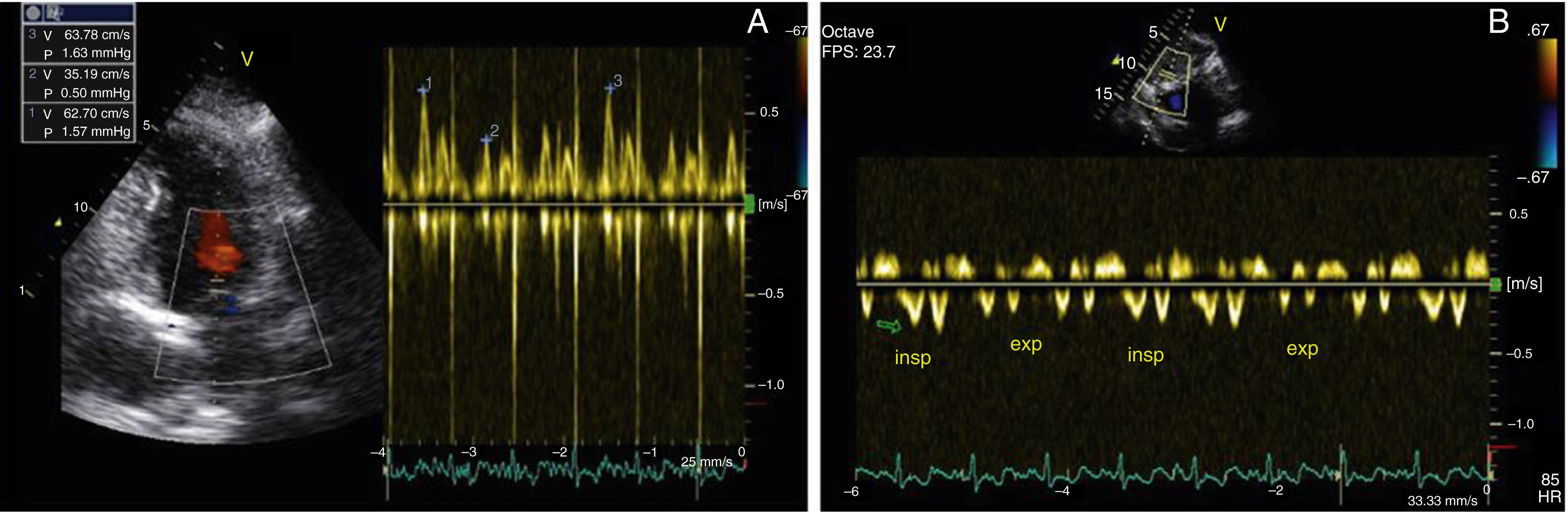
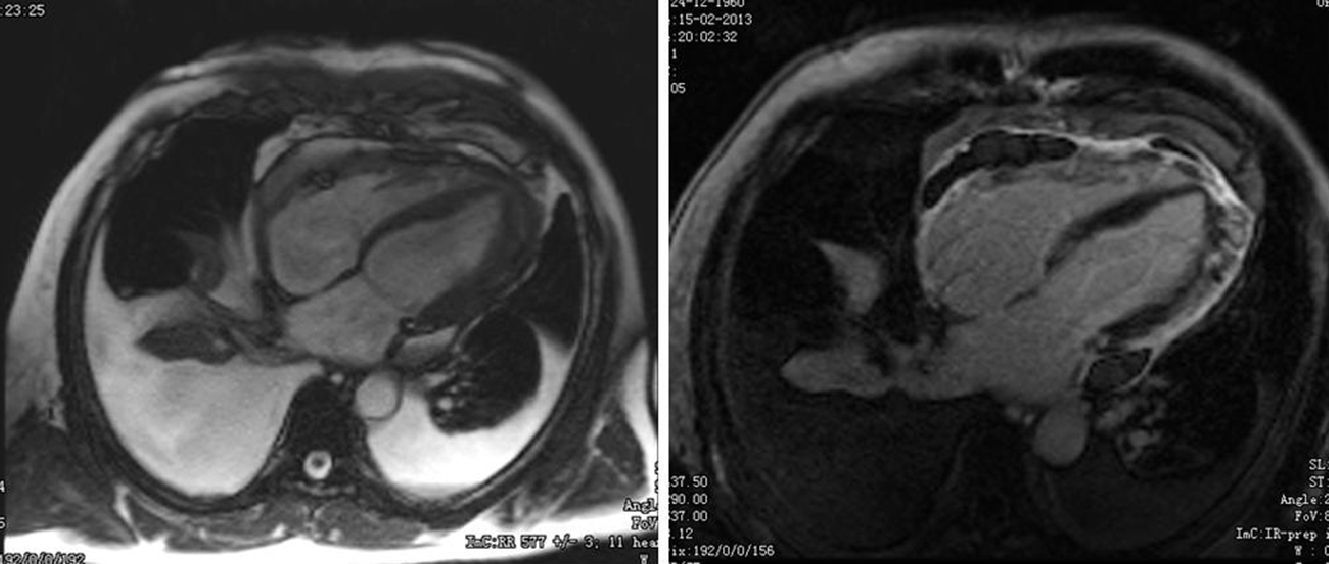
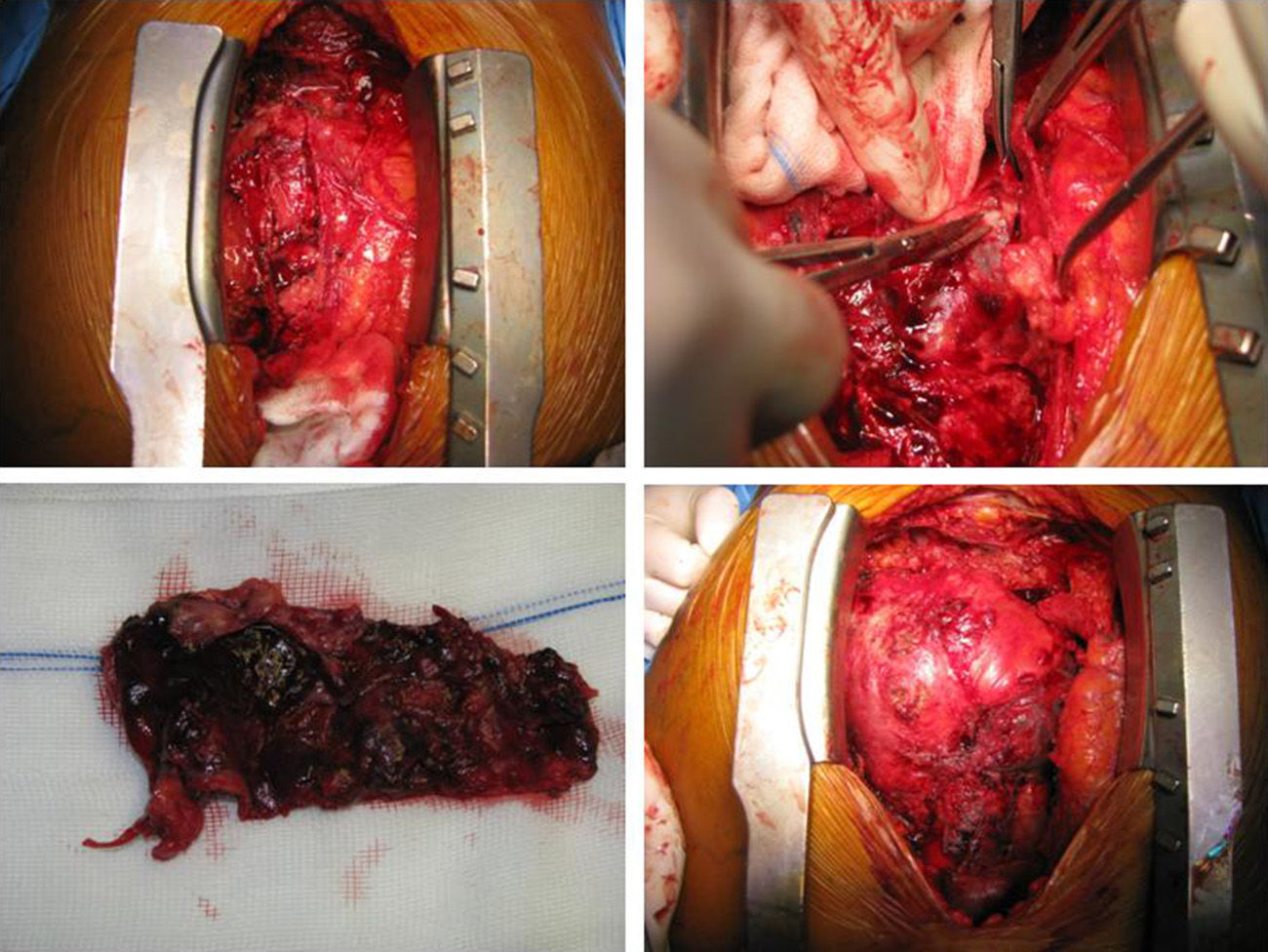
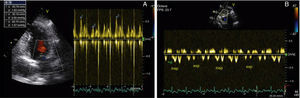
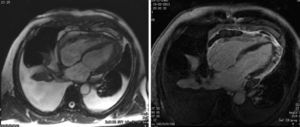
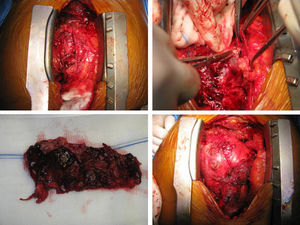
After two months of antituberculosis therapy, repeat echocardiography showed signs of pericardial constriction (septal bounce and significant respiratory variation in cardiac valve and hepatic vein flows – Figure 3), together with a thin rim of pericardial effusion. CMR confirmed these findings and revealed pericardial thickening, with no signal intensification after contrast injection and an extensive area of late enhancement, as well as areas of pericardia-myocardial adherence and signs of constrictive physiology (septal bounce) (Figure 4). Clinically, predominantly right-sided heart failure was observed during the third month of medical therapy, prompting rehospitalization; this rapidly worsened and the patient developed anasarca, liver failure, exudative enteropathy and cachexia. In view of the diagnosis of effusive-constrictive pericarditis, with rapid and progressive clinical deterioration and signs of chronicity, he was referred for surgical pericardiectomy. Intraoperatively, complete pericardial symphysis was observed, with a macroscopic appearance suggestive of tumor infiltration (Figure 5). Partial decortication was performed, given the technical difficulties presented by the presence of areas of pericardia-myocardial adherence.
Postoperatively the patient developed biventricular failure, which did not respond to amine therapy, and he died on the sixth postoperative day. Anatomopathologic study of the surgical specimen revealed pericardial metastases suggestive of poorly differentiated squamous cell carcinoma, probably of pulmonary origin.
DiscussionConstrictive pericarditis should be considered in differential diagnosis of right heart failure. Its etiology has changed over time, particularly in developed countries.2 The most common etiologies are now idiopathic and cardiac surgery, followed by radiotherapy, connective tissue disorders, infection and cancer.2 Tuberculosis remains the main cause in developing countries and in immunosuppressed patients.3 Pericardial involvement due to cancer is usually manifested by pericardial effusion, mainly secondary to metastases arising from lung (35%) or breast cancer (25%), lymphoma or leukemia (15%). Pericardial effusion secondary to lung cancer or to solid tumors other than breast has a worse prognosis than that caused by breast or blood cancers.4
Beside the classic form, constrictive pericarditis may present as localized, transient or occult constriction or be effusive-constrictive,5 in which the constrictive physiology is associated with pericardial effusion and persists after pericardiocentesis.6
Constrictive pericarditis is caused by fibrosis and calcification of the pericardium, which reduces its compliance, increases venous pressures and eventually restricts ventricular diastolic filling. This causes characteristic respiration-related hemodynamic changes. Left ventricular (LV) flow is reduced during inspiration as a result of two mechanisms: reduced pressure gradient between the pulmonary veins and the left atrium due to pericardial stiffness, which limits transmission of reduced intrathoracic pressure to the pericardial space; and increased ventricular interdependence, triggered by LV underfilling, which causes leftward deviation of the interventricular septum (IVS), leading to increased filling of the right chambers and pressure overload on the IVS.7
Echocardiography plays a crucial role in the diagnosis of constrictive pericarditis. Suggestive findings are: (1) pericardial thickening, although this may not always be present; (2) septal bounce; (3) dilatation and absent or diminished collapse of the inferior vena cava (IVC) and suprahepatic veins; and (4) preserved or increased mitral annular early diastolic velocity (e′), an important sign in differential diagnosis with restrictive cardiomyopathy, in which e′ is usually <7 cm/s.3 The hallmark of constrictive pericarditis on Doppler echocardiography is increased respiratory flow variation through the heart valves, expressed as a decrease in flow through the left-side valves during inspiration and an increase with expiration, the reverse occurring with the right-side valves. This sign is significant if the variation in the mitral E wave is at least 25%, which rarely occurs in restrictive cardiomyopathy.1
After echocardiography, CMR is the method of choice to study the pericardium. Due to its fibrous composition, healthy pericardium shows up as a hyperdense structure on T1- and T2-weighted sequences. The typical morphological characteristics of constrictive pericarditis are overall thickening of the pericardial layers (>4 mm) and late enhancement of the pericardium, associated with the acute phase of pericardial inflammation.8 CMR can also distinguish between small pericardial effusions and pericardial thickening, as well as detecting septal bounce and pericardia-myocardial adherence.2 Cardiac CT is also useful in diagnosing constrictive pericarditis as it can show pericardial thickening and calcification, as well as IVC dilatation and angulation of the IVS. Failure of the immediately adjacent pulmonary structures to pulsate during the cardiac cycle in the presence of a thickened pericardium is highly suggestive of a constrictive physiology.3 These two diagnostic techniques are particularly valuable if respiratory variation on echocardiography is inconclusive, or when other clinical conditions are present that can be associated with increased respiratory variation in transvalvular flows, such as obesity or chronic obstructive pulmonary disease.9
Invasive hemodynamic assessment by cardiac catheterization may be necessary for a diagnosis of constrictive pericarditis, depending on the results of noninvasive imaging modalities. There is a characteristic equalization of diastolic pressures in all four chambers, generally with a difference of <5 mmHg.1 Ventricular pressures typically show a dip-and-plateau pattern, also known as the square root sign (rapid early diastolic ventricular filling, which then ceases).
Although constrictive pericarditis is usually a chronic condition, it can be transient (except in cases secondary to radiotherapy). In the absence of evidence of chronicity (cachexia, atrial fibrillation, liver failure and pericardial calcification), some authors argue that treatment should comprise medical therapy and clinical surveillance for two to three months before surgical pericardiectomy is considered.10 However, patients should be closely monitored, since pericardiectomy should be performed as soon as signs of chronic constriction develop, given that survival is better in patients undergoing pericardiectomy than in those treated conservatively.11 Nevertheless, the timing of surgery is critical, since pericardiectomy has little or no benefit in cases of advanced constriction, and surgical risk in these patients is unacceptably high.1 In the case of effusive-constrictive pericarditis, visceral as well as parietal pericardiectomy is required since the cause of the constriction is the visceral pericardium, which increases the technical complexity of the procedure.1,6
In the case presented, the only positive finding in the diagnostic process pointed to a diagnosis of tuberculous pericarditis, and medical therapy was begun. During clinical and echocardiographic follow-up, signs of pericardial constriction and rapidly worsening right heart failure were detected, followed by rapid progression to anasarca, liver failure, exudative enteropathy and cachexia. The patient underwent pericardiectomy and the macroscopic appearance of the pericardium was suggestive of a neoplastic etiology. This was completely unexpected in the light of the preoperative exams, but was subsequently confirmed by anatomopathologic study.
This case report highlights the fact that diagnosis of constrictive pericarditis remains a clinical challenge and requires a combination of invasive and noninvasive diagnostic techniques for differential diagnosis and thorough investigation of the underlying cause. Most importantly, these patients need to be closely monitored for early detection of signs of chronicity, which requires prompt referral for pericardiectomy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Marta L, Alves M, Peres M, et al. Pericardite efusiva-constritiva como manifestação de um diagnóstico inesperado. Rev Port Cardiol. 2014. http://dx.doi.org/10.1016/j.repc.2014.08.013