To estimate the cost-effectiveness and cost-utility of ticagrelor in the treatment of patients with acute coronary syndromes (unstable angina or myocardial infarction with or without ST-segment elevation), including patients treated medically and those undergoing percutaneous coronary intervention or coronary artery bypass grafting.
MethodsA short-term decision tree and a long-term Markov model were used to simulate the evolution of patients’ life-cycles. Clinical effectiveness data were collected from the PLATO trial and resource use data were obtained from the Hospital de Santa Marta database, diagnosis-related group legislation and the literature.
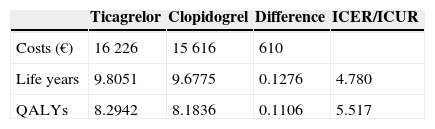
ResultsTicagrelor provides increases of 0.1276 life years and 0.1106 quality-adjusted life years (QALYs) per patient. From a societal perspective these clinical gains entail an increase in expenditure of €610. Thus the incremental cost per life year saved is €4780 and the incremental cost per QALY is €5517.
ConclusionsThe simulation results show that ticagrelor reduces events compared to clopidogrel. The costs of ticagrelor are partially offset by lower costs arising from events prevented. The use of ticagrelor in clinical practice is therefore cost-effective compared to generic clopidogrel.
Estimar os rácios custo-efetividade e custo-utilidade da utilização de ticagrelor versus clopidogrel no tratamento de doentes com síndromas coronárias agudas (angina instável, enfarte do miocárdio sem elevação ST [NSTEMI] ou enfarte do miocárdio com elevação ST [STEMI]); incluindo doentes sujeitos a tratamento médico e aqueles geridos com intervenção coronária percutânea (ICP) ou bypass aortocoronário com enxerto (CABG).
MetodologiaFoi utilizada uma árvore de decisão de curto prazo e um modelo de Markov de longo prazo para simular a progressão dos doentes no decurso da sua vida. Os dados de eficácia clínica foram recolhidos a partir do ensaio clínico PLATO e os dados de consumo de recursos foram obtidos na Contabilidade Analítica do Hospital de Santa Marta, legislação dos GDH e consulta de bibliografia disponível.
ResultadosTicagrelor proporciona, a cada doente, um incremento de 0,1276 anos de vida e 0,1106 QALY. Na perspectiva da sociedade, estes ganhos implicam um aumento da despesa em 610€. Obtêm-se, assim, um custo incremental por ano de vida salvo de 4780€ e um custo incremental por QALY de 5517€.
ConclusõesOs resultados obtidos mostram que o ticagrelor diminui a quantidade de eventos, quando comparado com clopidogrel. Os custos com ticagrelor são parcialmente compensados por uma diminuição dos custos decorrentes dos eventos evitados. Assim, a utilização de ticagrelor na prática clínica portuguesa é custo-efetiva quando comparado com a abordagem com clopidogrel.
acute coronary syndromes
adenosine diphosphate
coronary artery bypass grafting
diagnosis-related group
incremental cost-effectiveness ratio
incremental cost-utility ratio
myocardial infarction
non-ST-segment myocardial infarction
percutaneous coronary intervention
Portuguese Registry on Acute Coronary Syndromes
quality-adjusted life year
ST-segment elevation myocardial infarction
unstable angina
value-added tax
Cardiovascular disease is the leading cause of death in Portugal, accounting for 32.3% of all deaths in 2007 according to a 2008 report from the Portuguese National Institute of Statistics. According to the Portuguese High Commissariat for Health in 2007, of cardiovascular deaths, 44.9% were due to cerebrovascular disease and 23.1% to ischemic heart disease, particularly acute coronary syndromes (ACS).
ACS represent a life-threatening manifestation of atherosclerosis usually precipitated by acute thrombosis, induced by a ruptured or eroded atherosclerotic plaque, with or without concomitant vasoconstriction, causing a sudden and critical reduction in blood flow. This triggers a cascade of reactions resulting in the formation of a coronary thrombus that completely or partially obstructs the arterial lumen.1,2
ACS are a group of clinical conditions, all of which share a common pathophysiological substrate, that of unstable atherothrombotic coronary disease: ST-segment elevation myocardial infarction (MI) (STEMI), non-ST-segment MI (NSTEMI), and unstable angina (UA).1,2
The Portuguese Registry on Acute Coronary Syndromes (ProACS), the main source of information on ACS in Portugal, was established in 2002 with the purpose of analyzing the clinical characteristics, treatment and outcomes of ACS patients, monitoring changes over time, and assessing compliance with the guidelines on ACS. A total of 22482 patients were included between 2002 and 2009, with a mean age of 66±13 years, 70% male, of whom 45.4% were diagnosed with STEMI, 41.4% with NSTEMI and 13.1% with UA.3
Platelet activation plays an important part in the pathophysiology of ACS, not only in cases of acute plaque rupture, but also as a contributing factor for subsequent atherothrombotic events in the systemic circulation of patients with inflammation of the arterial wall. There are three main types of antiplatelet drug: cyclooxygenase inhibitors (most commonly aspirin); adenosine diphosphate (ADP) receptor inhibitors (the thienopyridines ticlopidine, clopidogrel, and prasugrel); and glycoprotein IIb/IIIa inhibitors, including tirofiban, eptifibatide, and abciximab.4
Ticagrelor (trade name Brilique® in Europe) is a non-heparin antiplatelet drug that is the first orally active reversible P2Y12 receptor antagonist of a new chemical class, the cyclopentyl-triazolo-pyrimidines.5 Like the thienopyridines, ticagrelor blocks P2Y12, an ADP receptor, and thereby inhibits ADP-mediated platelet activation and aggregation. However, unlike thienopyridines, which are irreversible, ticagrelor binds directly and reversibly to P2Y12 and non-competitively inhibits signal transduction.
MethodsThe present study estimates the cost-effectiveness and cost-utility of ticagrelor (loading dose, 90 mg twice daily thereafter) vs. clopidogrel (loading dose, 75 mg daily thereafter) in the treatment of patients with ACS.
The effects were measured in life years saved and quality-adjusted life years (QALYs). Clinical effectiveness data were collected from the PLATO trial. The main endpoints were death from any cause, MI and non-fatal stroke.
The main characteristics of the population used in the model, based on the PLATO trial, are shown in Table 1.
The time horizon was set at a patient's lifetime in order to assess the long-term impact of therapy on health outcomes and associated costs.
The analysis was conducted from a societal perspective in accordance with the Portuguese Ministry of Health's guidelines for economic evaluation studies of drugs,6 in which the costs and effects for all parties involved are taken into account, including indirect costs.
Clinical effectivenessPLATO7 was a multicenter, double-blind, randomized trial comparing ticagrelor (180-mg loading dose, 90 mg twice daily thereafter) and clopidogrel (300–600-mg loading dose, 75 mg daily thereafter) for the prevention of cardiovascular events in 18624 patients hospitalized with ACS with or without ST-segment elevation.
The primary endpoint was a composite of death from vascular causes, MI, or stroke, measured as the number of events. At 12 months, the primary endpoint had occurred in 9.8% of patients receiving ticagrelor compared with 11.7% of those receiving clopidogrel (hazard ratio [HR], 0.84; 95% confidence interval, 0.77–0.92; p<0.001).
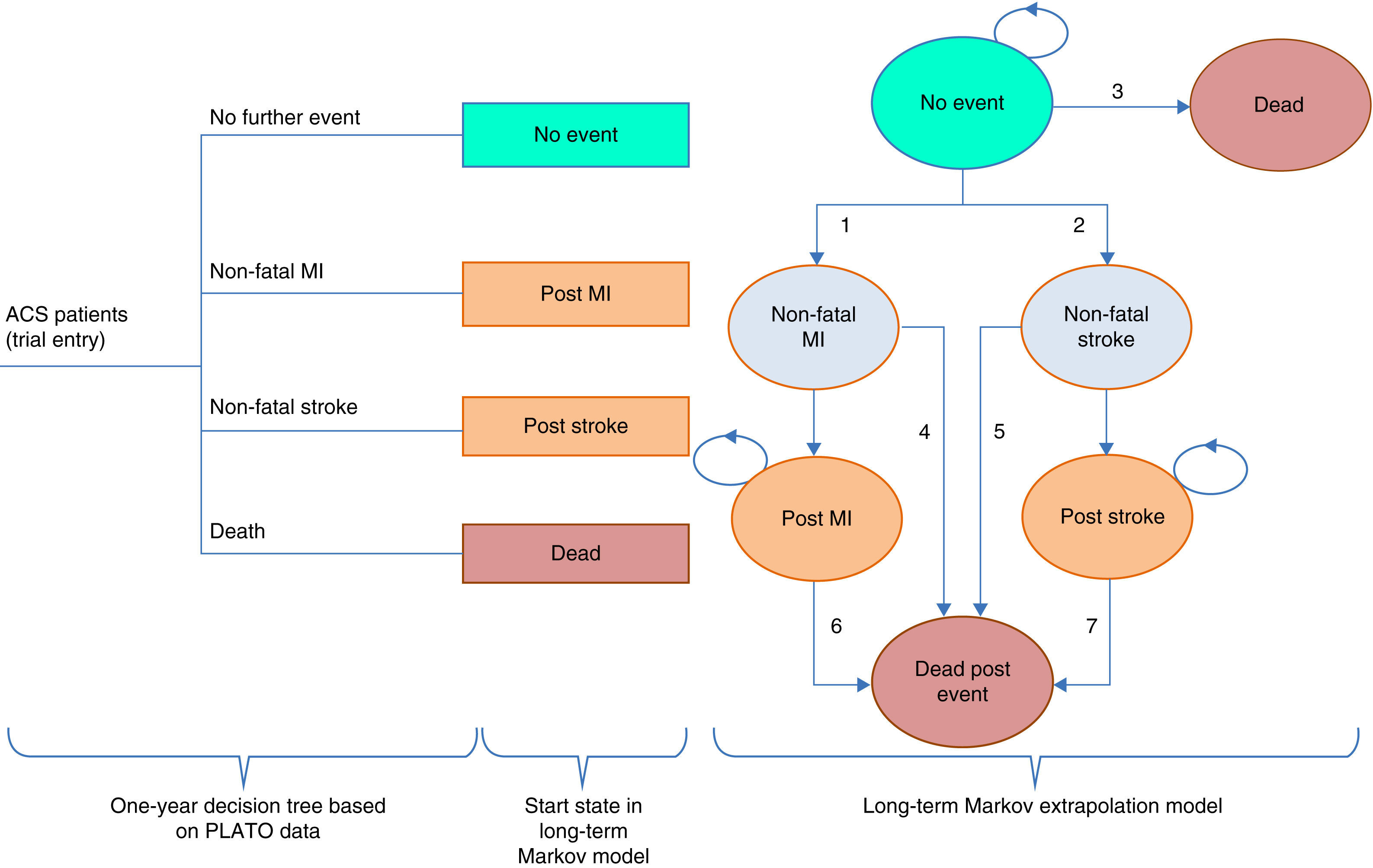
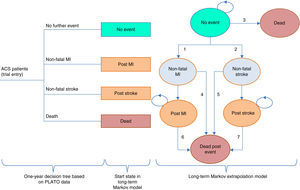
ModelThe model used to evaluate ticagrelor was adapted for the Portuguese situation from a European model (Nikolic et al.8). This model, which has been the subject of several publications, consists of two parts: a short-term decision tree for the first year based on data from the PLATO trial, and a Markov model for long-term extrapolation, in order to cover all the major events associated with resource use and clinical outcomes during a patient's lifetime. The model is similar to other decision analysis models on ACS.9–11
Figure 1 illustrates the model structure.
Model structure (adapted from Nicolic et al.8). ACS: acute coronary syndrome; MI: myocardial infarction.
In the first year, patients are allocated to nodes in the decision tree according to the estimated probability of suffering each event, which differs between the two treatment strategies according to the treatment effect observed in the PLATO trial. Each node is assigned estimates of health care use and QALYs.
After the first year, it is assumed that patients are no longer on treatment with ticagrelor or clopidogrel, without direct repercussions (treatment effects or post-treatment relapse) for the cycles of the Markov model.
The Markov cycles have a duration of one year. Each health state is associated with an estimate of health-related quality of life expressed in QALYs and of health resource use. The patient cohort progresses through the Markov model according to the estimated probability of transition based on the PLATO data.
In each year patients without events have a risk of suffering non-fatal MI or non-fatal stroke (transitions 1 and 2); in the case of a non-fatal event, patients progress to the “Non-fatal MI” or “Non-fatal stroke” state. Patients at the “No event” state are also at risk of death (transition 3) every year, and if this occurs, they progress to the “Death” state, an absorbing state following which no further transitions are possible.
The states “Non-fatal MI” and “Non-fatal stroke” represent the first-year outcome in terms of survival, costs and quality of life of patients who suffered a non-fatal event, but who are still at risk of dying and thus moving to the “Dead post event” state (transitions 4 and 5); the “Non-fatal MI” and “Non-fatal stroke” states are tunnel states, meaning that patients can only remain in them for one cycle. Patients who are still alive after a year pass to the “Post MI” or “Post stroke” states, which like the “Non-fatal MI” and “Non-fatal stroke” states represent patients’ outcomes in terms of survival, costs and quality of life in the second and subsequent years after a non-fatal event following entry to the PLATO trial.
The “Post MI” and “Post stroke” states are associated with risk of death (transitions 6 and 7) as well as with costs and QALYs.
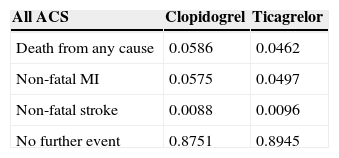
Short-term calibration of the modelFor short-term calibration of the model, the probabilities were based on data from the PLATO trial (Table 2). Four transition probabilities were obtained to calibrate the decision tree nodes:
- 1.
The probability of non-fatal MI;
- 2.
The probability of non-fatal stroke;
- 3.
The probability of death from any cause;
- 4.
The probability of having no further event, defined as the difference between 1 and the sum of the probabilities of the other three events.
At the end of the first year, there are differences between patients treated with ticagrelor and clopidogrel, reflected in the distribution between the different health states. In the long-term model no patients are receiving either medication and so the transition probabilities are the same for both arms. Even so, the long-term treatment effects differ as a function of the different starting points.
The annual mortality risks in the “No event” state (transition 3) are estimated using age-specific mortality rates taken from Portuguese life tables.12 It should be borne in mind that patients in this state have been event-free for at least a year since the initial ACS, and the evidence suggests that ACS patients are at greater risk of suffering a fatal event in the following 12 months, the risk decreasing thereafter.13,14 An HR of 2 was applied to mortality rates for age and gender to incorporate the increased risk associated with ACS. A standard deviation of 0.10 was assumed for the logarithm of the HR for the probabilistic sensitivity analysis and the ratio was assumed to follow a normal distribution on the logarithmic scale.
For the “Non-fatal MI” state (transition 4), the mortality risk observed in the PLATO trial was compared to the risk in the life tables and an HR of 6 was applied. A standard deviation of 0.3 was assumed for the logarithm of the HR for the probabilistic sensitivity analysis and this ratio was assumed to follow a normal distribution on the logarithmic scale.
For the “Non-fatal stroke” state (transition 5), the relative mortality risk in the first year after ACS compared with age- and gender-matched individuals in the general population was based on the results of three studies.15–17 The study by Dennis et al.,15 with a risk of 7.43, was considered the most robust since it had the longest follow-up and the largest number of patients. As there were more cases of ACS in the ticagrelor group, it was decided to use 7.43 as a conservative figure in the analysis of this treatment. A standard deviation of 0.35 on the logarithmic scale was assumed.
For the transition from “Post MI” to “Dead” (transition 6), it was assumed that patients in the “Post MI” state, having suffered reinfarction, are at higher risk than patients in the “No event” state. Patients in the “Post MI” state have survived a year after the recurrent event and have a lower mortality risk than those in the “Non-fatal MI” state. It is therefore likely that the risk of patients in the “Post MI” state is between that of the “No event” and “Non-fatal MI” states. An HR of 3 was accordingly applied to maintain the logical consistency of the values in the model's parameters. A standard deviation of 0.15 on the logarithmic scale was assumed for the probabilistic sensitivity analysis.
For the “Post stroke” state (transition 7), the studies used are highly consistent in their attribution of mortality risk in the second and subsequent years after the initial stroke.18 The figure of 2.07, from Dennis et al.,15 was taken as the relative mortality risk in the first year, while for the second and subsequent years it was taken as the mean of the other years of follow-up, which was consistent from the second year onwards. A standard deviation of 0.10 on the logarithmic scale was assumed for the probabilistic sensitivity analysis.
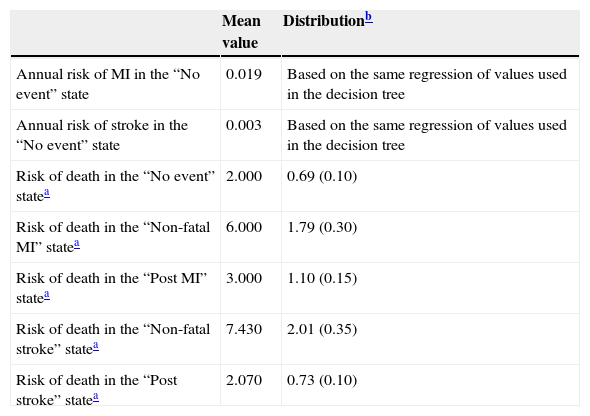
The transition parameters of the Markov model in the base case are summarized in Table 3.
Transition parameters of the Markov model.
| Mean value | Distributionb | |
|---|---|---|
| Annual risk of MI in the “No event” state | 0.019 | Based on the same regression of values used in the decision tree |
| Annual risk of stroke in the “No event” state | 0.003 | Based on the same regression of values used in the decision tree |
| Risk of death in the “No event” statea | 2.000 | 0.69 (0.10) |
| Risk of death in the “Non-fatal MI” statea | 6.000 | 1.79 (0.30) |
| Risk of death in the “Post MI” statea | 3.000 | 1.10 (0.15) |
| Risk of death in the “Non-fatal stroke” statea | 7.430 | 2.01 (0.35) |
| Risk of death in the “Post stroke” statea | 2.070 | 0.73 (0.10) |
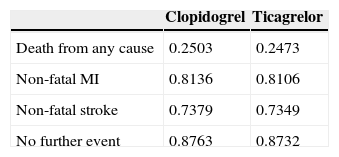
The estimates of utility used in each node of the decision tree were based on EQ-5D questionnaire data11 collected within the PLATO study.
The mean QALYs for each node were estimated using ordinary least squares regression with the nodes and treatment groups introduced as dummy variables. The equations include age and gender as explanatory variables to allow estimates of QALYs for different ages and for men and women separately.
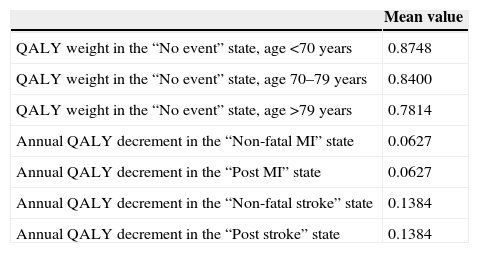
The mean estimated QALYs for each node and for each treatment group are presented in Table 4.
Utility in the long-term modelFor the “No event” state the estimate of QALYs for event-free patients in the PLATO trial was used. The mean estimate of ticagrelor- and clopidogrel-treated patients (0.875) was applied for patients aged <70 years. When men and women were analyzed separately, the estimated QALYs for men were 0.05 higher for men than for women; the estimate also varies with age at trial entry, and as patients grow older in the model, a proportional decrement due to age was applied.
The QALY estimates for the Markov model are summarized in Table 5.
QALY estimates for the Markov model.
| Mean value | |
|---|---|
| QALY weight in the “No event” state, age <70 years | 0.8748 |
| QALY weight in the “No event” state, age 70–79 years | 0.8400 |
| QALY weight in the “No event” state, age >79 years | 0.7814 |
| Annual QALY decrement in the “Non-fatal MI” state | 0.0627 |
| Annual QALY decrement in the “Post MI” state | 0.0627 |
| Annual QALY decrement in the “Non-fatal stroke” state | 0.1384 |
| Annual QALY decrement in the “Post stroke” state | 0.1384 |
MI: myocardial infarction.
The model design only allows indirect costs to be added to events in the first year, which means the effects of morbidity on the population's productivity are underestimated, since some events will not occur until after the first year.
The estimated mean annual productivity in 2010 for those aged >60 was €15743 for men and €10839 for women, corresponding to mean daily productivity of €68.50 and €47.12, respectively. These figures were not updated for 2012, which is reasonable given the state of the labor market and salaries in Portugal in recent years.
The absenteeism associated with an event is taken to be the number of working days equal to the mean hospital stay plus twice this number for convalescence. On the basis of the diagnosis-related groups (DRGs) in force in 2009, the mean hospital stay for stroke survivors was calculated as 10.3 days and for MI survivors as 7.9 days. Absenteeism is thus 30.9 days for stroke and 23.7 days for MI, the corresponding costs being calculated as the product of days lost, daily cost, and post-event employment rates.
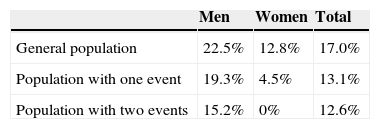
The employment rates used in the calculations are shown in Table 6.
The cost of lost employment due to early retirement was based on the assumption that leaving the labor market reduces productivity by at least 2.5 years on average, corresponding to an example in which the mean age for the analysis is taken to be 62.5 years and normal retirement is at age 65.
Costs of ticagrelor and the comparator clopidogrelTreatment with ticagrelor should begin with a single loading dose of 180 mg (two 90-mg tablets) followed by 90 mg twice daily. The daily cost of ticagrelor is thus €2.67 (retail price without value added tax [VAT]).
The daily cost of clopidogrel, which at the time of the analysis was €0.32, was estimated on the basis of 75 mg daily at the reference price of the corresponding ‘homogeneous group’ of medications (GH0736), without VAT.
Other direct costsOther direct costs, including those related to the use of health care services (hospitalization, consultations, other medications, diagnostic exams, other treatments, etc.), were estimated separately for each node of the decision tree (first year) and subsequent cycles of the Markov model.
For the first year, Portuguese unit costs and the resource use data from the PLATO study were used, while updated estimates of the direct costs generated in the first year were used for subsequent cycles.
In the event of differences between the estimates based on PLATO data and more precise estimates based on typical resource use patterns in the Portuguese health system, a sensitivity analysis was performed for the parameters in question.
Estimates of unit costs are generally based on the DRGs in force and audit information from the cardiology and cardiothoracic surgery departments of Hospital de Santa Marta.
The results are shown in Table 7.
Costs in the first year in the base case.
| Events in the first year | Costs (€) |
|---|---|
| Clopidogrel group, no event | 7656 |
| Ticagrelor group, no event | 7456 |
| Clopidogrel group, MI | 15258 |
| Ticagrelor group, MI | 15058 |
| Clopidogrel group, stroke | 14943 |
| Ticagrelor group, stroke | 14742 |
| Clopidogrel group, death | 12291 |
| Ticagrelor group, death | 12091 |
MI: myocardial infarction.
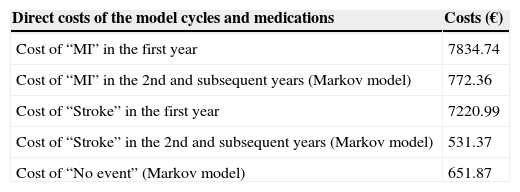
For subsequent cycles, updated estimates of the direct costs generated in the first year and for following years were used for MI and stroke (Table 8).
Direct costs after the first year in the base case.
| Direct costs of the model cycles and medications | Costs (€) |
|---|---|
| Cost of “MI” in the first year | 7834.74 |
| Cost of “MI” in the 2nd and subsequent years (Markov model) | 772.36 |
| Cost of “Stroke” in the first year | 7220.99 |
| Cost of “Stroke” in the 2nd and subsequent years (Markov model) | 531.37 |
| Cost of “No event” (Markov model) | 651.87 |
MI: myocardial infarction.
The model's time horizon was set at a patient's lifetime and an annual discount rate of 5% was applied, in accordance with the guidelines for economic evaluation studies in Portugal.6
Univariate sensitivity analysisA sensitivity analysis is designed to determine the robustness of the results in relation to the assumptions made in the analysis when the available information is uncertain. A univariate sensitivity analysis was performed for the time horizon, exclusion of indirect costs, alternative discount rates and alternative costings for events. This analysis showed that the cost-effectiveness and cost-utility values were robust, since in all scenarios the incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR) were acceptable (less than €20000/QALY).
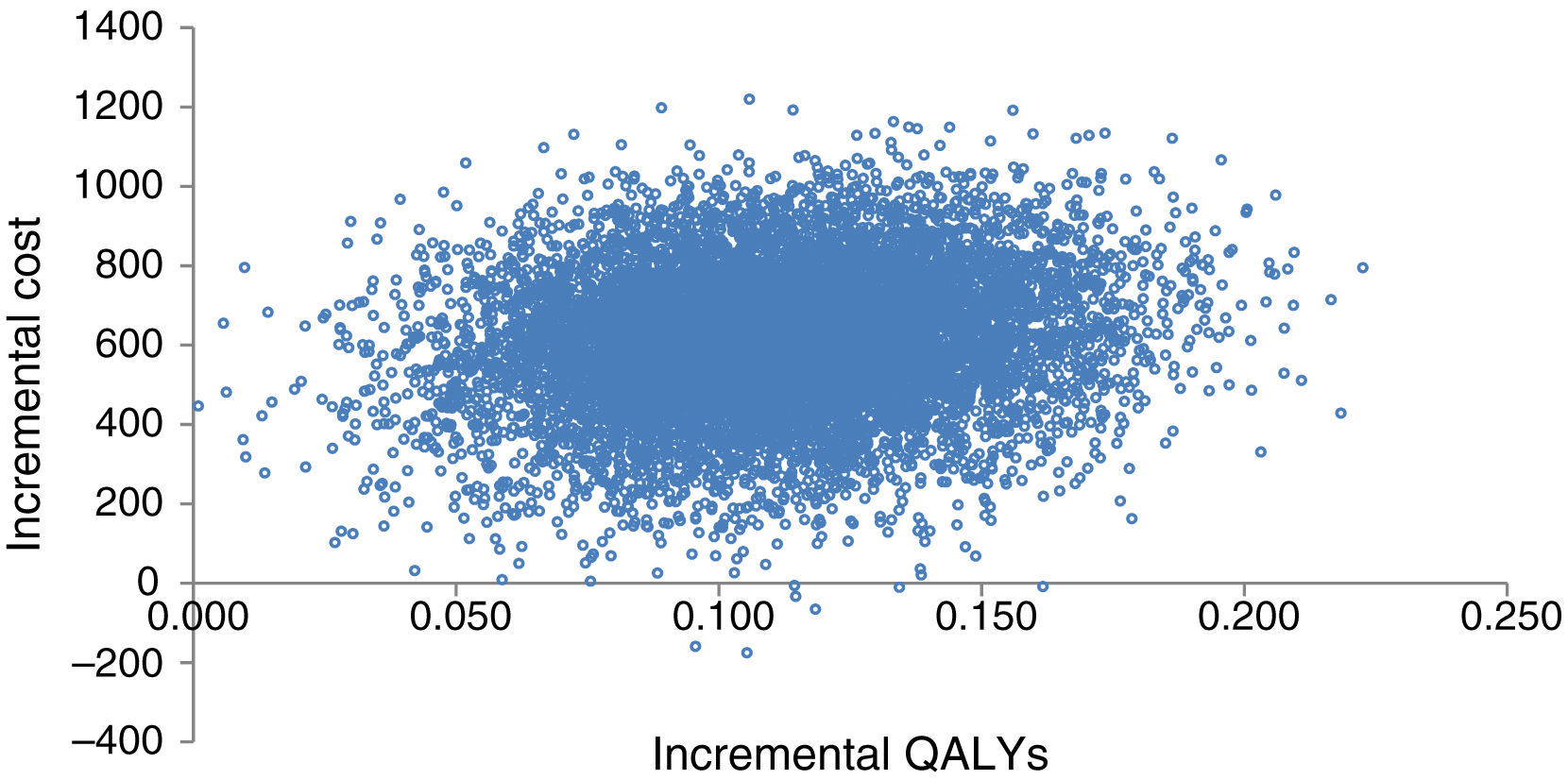
Probabilistic sensitivity analysisA second-order Monte Carlo simulation was used to account for the uncertainty arising from single model inputs, so that the uncertainty of the cost-effectiveness analysis reflects the uncertainty in the decision to implement a given treatment rather than the uncertainty of the model inputs.19,20 The distribution of incremental costs and QALYs is represented graphically in Figure 2, which is based on 10000 simulations of the model.
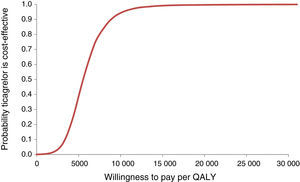
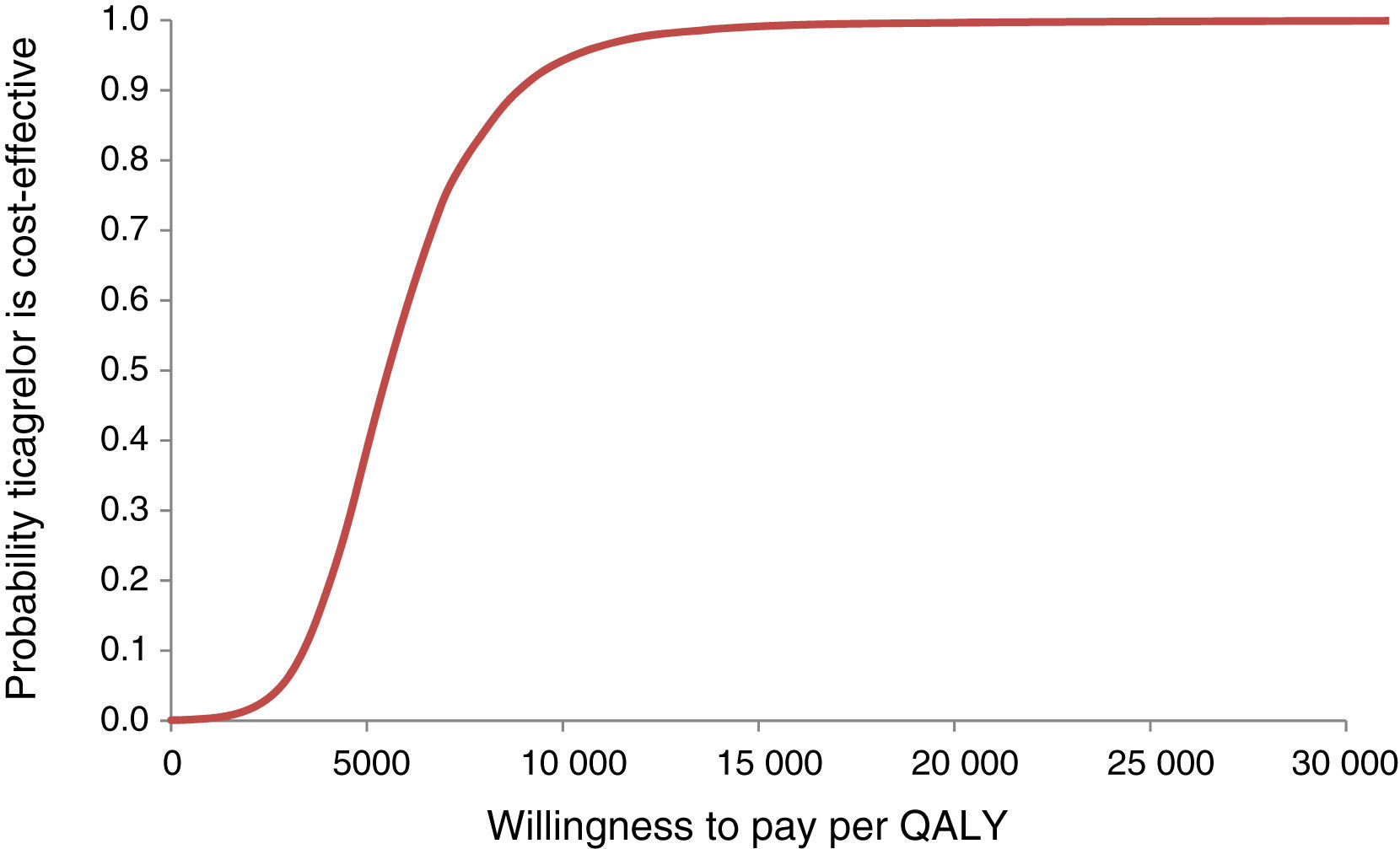
The probabilities of ticagrelor being cost-effective at different levels of willingness to pay for one QALY (acceptability curve) were also estimated (Figure 3).
The mean ICUR in the simulation was €5517/QALY; for a willingness to pay of €5541/QALY there is a 50% probability of acceptance. The 95% confidence intervals are €2314/QALY for 2.5 percentile and €11790/QALY for 97.5 percentile, well below the usual acceptability threshold of €20000/QALY.
DiscussionThe results were robust in a variety of univariate analyses and alternative scenarios. In some cases, when the sources of cost estimates of events were changed, such as excluding indirect costs, the incremental ratios are higher, but only slightly (2.4%). When the time horizon is reduced the incremental ratios are up to twice as high as the base case. In these cases the ICERs and ICURs change significantly, but even in the worst scenarios they remain less than €20000/QALY (Table 9).
Results of cost-effectiveness and cost-utility analysis.
| Ticagrelor | Clopidogrel | Difference | ICER/ICUR | |
|---|---|---|---|---|
| Costs (€) | 16226 | 15616 | 610 | |
| Life years | 9.8051 | 9.6775 | 0.1276 | 4.780 |
| QALYs | 8.2942 | 8.1836 | 0.1106 | 5.517 |
ICER: incremental cost-effectiveness ratio; ICUR: incremental cost-utility ratio; QALY: quality-adjusted life year.
All the results of this economic evaluation comparing ticagrelor and clopidogrel for secondary prevention following ACS show that ticagrelor has excellent levels of cost-effectiveness, producing health gains at costs well below the acceptable thresholds for willingness to pay on the part of the Portuguese National Health Service.
The results were robust in a variety of univariate analyses and alternative scenarios.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe economic evaluation was funded by AstraZeneca.
Conflicts of interestThe authors have no conflicts of interest to declare. AstraZeneca funded the adaptation of the original study to Portugal. The funding body had no influence on the authors of the study that affected the results obtained.
Please cite this article as: Gouveia M, Borges M, Trindade R, Rikner K. Avaliação económica de ticagrelor em prevenção secundária pós Síndroma Coronário Agudo. Rev Port Cardiol. 2015. http://dx.doi.org/10.1016/j.repc.2014.08.004



 ACS: acute coronary syndrome; MI: myocardial infarction.' title='Model structure (adapted from Nicolic et al.8).
ACS: acute coronary syndrome; MI: myocardial infarction.' title='Model structure (adapted from Nicolic et al.8). 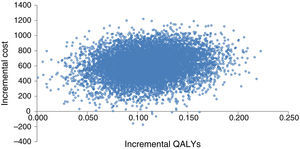 QALYs.
QALYs.