Metabolic syndrome is currently a worldwide epidemic. First-line interventions consist of profound changes in lifestyle, particularly in diet, for which moderate and balanced consumption of macronutrients associated with calorie restriction is recommended. However, in recent years, several studies have proposed that diets that restrict certain macronutrients even further may be better for controlling both weight and metabolic risk factors. In the present paper, we review the currently available evidence on this question, which is difficult to address and control in clinical practice.
A síndrome metabólica (SM) é hoje uma verdadeira epidemia a nível mundial. As intervenções de primeira linha passam por uma profunda modificação de estilo de vida, em particular dietéticos, recomendando-se consumo moderado e equilibrado de macronutrientes associado a restrição calórica. Contudo, nos últimos anos, diversos estudos têm vindo a sugerir que dietas com restrições mais intensas de alguns macronutrientes poderiam ser mais vantajosas quer para controlo ponderal quer no controlo dos fatores de risco metabólicos. Neste artigo, revemos a evidência acumulada até ao momento sobre essa problemática tão atual e que permanece difícil de abordar e controlar na prática clínica.
Metabolic syndrome (MS) is a clustering of various metabolic risk factors associated with insulin resistance. It has reached epidemic proportions worldwide, although with regional variations resulting from differences in socioeconomic conditions and in local ethnic and genetic characteristics, as well as in the definitions of MS used. The first definition was proposed by the World Health Organization in 1998,1 since when various other scientific bodies, including the US National Cholesterol Education Program, the International Diabetes Federation, and the American Heart Association/National Heart, Lung, and Blood Institute have each put forward their own definitions, with progressively simpler criteria.2–4 However, the proposed criteria are not consistent, which hinders comparisons between published studies of MS prevalence. For example, in one study of a single patient population using only the three most recent definitions, the prevalence of MS ranged between 39% and 54% depending on the definition used, with an overall agreement between the three of only 43.5%.5
In view of this inconsistency, in 2009 a group of scientific organizations arrived at a consensus and published a single definition of MS. This was based on previous definitions, the main difference between which concerned the measure for central obesity, specifically the definition of increased waist circumference. A fixed cut-point for the latter measure was not specified in the new document,6 which stated that national or regional cut-points could be used, bearing in mind ethnic and other local considerations. Thus a particular health system might adopt different cut-points locally for pragmatic or economic reasons, in view of the medical resources that would be consumed.
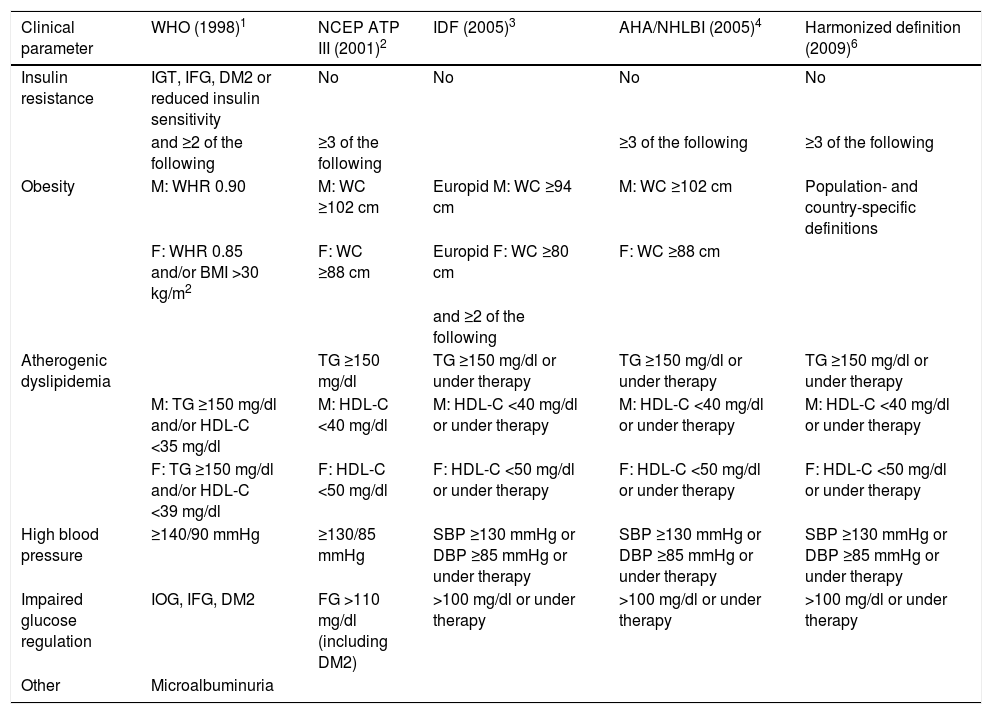
On the basis of this harmonized definition, MS is defined by the presence of three of the five criteria displayed in Table 1.
Most frequently used definitions of metabolic syndrome.
| Clinical parameter | WHO (1998)1 | NCEP ATP III (2001)2 | IDF (2005)3 | AHA/NHLBI (2005)4 | Harmonized definition (2009)6 |
|---|---|---|---|---|---|
| Insulin resistance | IGT, IFG, DM2 or reduced insulin sensitivity | No | No | No | No |
| and ≥2 of the following | ≥3 of the following | ≥3 of the following | ≥3 of the following | ||
| Obesity | M: WHR 0.90 | M: WC ≥102 cm | Europid M: WC ≥94 cm | M: WC ≥102 cm | Population- and country-specific definitions |
| F: WHR 0.85 and/or BMI >30 kg/m2 | F: WC ≥88 cm | Europid F: WC ≥80 cm | F: WC ≥88 cm | ||
| and ≥2 of the following | |||||
| Atherogenic dyslipidemia | TG ≥150 mg/dl | TG ≥150 mg/dl or under therapy | TG ≥150 mg/dl or under therapy | TG ≥150 mg/dl or under therapy | |
| M: TG ≥150 mg/dl and/or HDL-C <35 mg/dl | M: HDL-C <40 mg/dl | M: HDL-C <40 mg/dl or under therapy | M: HDL-C <40 mg/dl or under therapy | M: HDL-C <40 mg/dl or under therapy | |
| F: TG ≥150 mg/dl and/or HDL-C <39 mg/dl | F: HDL-C <50 mg/dl | F: HDL-C <50 mg/dl or under therapy | F: HDL-C <50 mg/dl or under therapy | F: HDL-C <50 mg/dl or under therapy | |
| High blood pressure | ≥140/90 mmHg | ≥130/85 mmHg | SBP ≥130 mmHg or DBP ≥85 mmHg or under therapy | SBP ≥130 mmHg or DBP ≥85 mmHg or under therapy | SBP ≥130 mmHg or DBP ≥85 mmHg or under therapy |
| Impaired glucose regulation | IOG, IFG, DM2 | FG >110 mg/dl (including DM2) | >100 mg/dl or under therapy | >100 mg/dl or under therapy | >100 mg/dl or under therapy |
| Other | Microalbuminuria |
AHA/NHLBI: American Heart Association/National Heart, Lung, and Blood Institute; BMI: body mass index; DBP: diastolic blood pressure; DM2: type 2 diabetes; F: females; FG: fasting glucose; HDL-C: high-density lipoprotein cholesterol; IDF: International Diabetes Federation; IFG: impaired fasting glycemia; IGT: impaired glucose tolerance; M: males; NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III; SBP: systolic blood pressure; TG: triglycerides; WC: waist circumference; WHO: World Health Organization; WHR: waist/hip ratio.
AHA/NHLBI: American Heart Association/National Heart, Lung, and Blood Institute; DM2: type 2 diabetes; BMI: body mass index; DBP: diastolic blood pressure; F: females; FG: fasting glucose; HDL-C: high-density lipoprotein cholesterol; IDF: International Diabetes Federation; IFG: impaired fasting glycemia; IGT: impaired glucose tolerance; M: males; NCEP ATP III: National Cholesterol Education Program Adult Treatment Panel III; SBP: systolic blood pressure; TG: triglycerides; WC: waist circumference; WHO: World Health Organization; WHR: waist/hip ratio.
Adipose tissue is an organ directly involved in whole-body lipid homeostasis. It is metabolically flexible, able to switch between storage and release of fatty acids according to metabolic needs.7 If the lipid storage capacity of adipose tissue is exceeded, then lipids are deposited in ectopic locations, causing peripheral insulin resistance, one of the main pathophysiological mechanisms involved in MS.8 Subcutaneous fat is considered ‘healthy’, unlike visceral adipose tissue, which is associated with significant inflammation, producing cytokines and adipokines that lead to systemic dysregulation that is responsible for many of the complications of MS.7 Insulin resistance causes hyperglycemia, resulting in glycation damage to vulnerable proteins including apolipoproteins, which disrupts their function and leads to changes in systemic lipid levels.8
Several meta-analyses in recent years have consistently demonstrated the adverse effects of MS in terms of cardiovascular events, including cardiac ischemic events and death as well as cerebrovascular events. Control of MS is thus a serious global health problem.9–11 However, unfortunately no specific treatment for MS is available. International guidelines identify interventions to reduce metabolic risk factors as the first-line treatment.4 These include not only specific treatment for each risk factor found in a particular individual, but more importantly, profound changes in lifestyle, particularly increasing exercise levels and changing eating habits. Dietary recommendations consist of moderate calorie restriction, with a moderate intake of the different macronutrients and only 25-35% of calories from fats, low levels of saturated fats and cholesterol, reduced intake of salt and simple sugars, and ample fruits, vegetables and whole grains.
However, some recent studies have indicated that different dietary compositions may be more beneficial in individuals with MS. Alternatives include low-fat diets (less than 30% of total energy intake), the main advantage of which is in lowering cholesterol levels, but which have the disadvantages of being less effective for weight control, requiring additional calorie restriction, being less flavorful, possibly causing vitamin and mineral deficiencies, and having higher proportions of sugars and carbohydrates. Another alternative is a low-carbohydrate diet (less than 50% of total energy intake) that is high in proteins and fats. This has the advantage of being more satisfying due to its high protein content, encouraging lower food intake, and does not require further calorie restriction, as well as presenting a low glycemic load. On the other hand, it involves high consumption of fats (frequently saturated) and may lack certain essential nutrients; meals take longer to prepare and may be less appetizing, hampering adherence, and the diet's low fiber content can lead to constipation, while its high protein content can result in ketosis and renal problems. Finally, mention should be made of the Mediterranean diet, the most balanced of these alternatives in terms of macronutrient composition, with a high proportion of plant-based foods, particularly vegetables, fruits, bread and unrefined cereals, pulses, legumes and nuts, and with an emphasis on locally-produced fresh and seasonal foods. The main source of fats is olive oil; dairy products, preferably low-fat, are consumed in moderation, while culinary herbs are used for seasoning instead of salt. Fish is a more frequent component of the Mediterranean diet than red meat, and wine is consumed in low to moderate quantities and only with main meals. Its main advantages over other diets are that it includes most macronutrients and its appetizing flavors improve long-term adherence. On the other hand, calorie restriction is still required for effective weight loss, while its high proportion of fresh foods involves higher costs and longer preparation times.
Small-scale clinical studiesSome small studies have provided evidence of the benefits of a low-carbohydrate diet in individuals with MS in terms of weight loss and metabolic risk factor control. Cornier et al.12 showed that insulin sensitivity in obese non-diabetic women determined the efficacy of low-calorie diets, with insulin-resistant obese women losing more weight under a low-carbohydrate diet, while insulin-sensitive women responded better to a low-fat diet. Regarding metabolic risk factors, in a study of individuals with MS by Volek et al.,13 a low-carbohydrate diet significantly improved not only weight and visceral adiposity but also blood pressure, lipid profile and fasting blood glucose, compared to a low-fat diet. A comparison of popular weight-loss diets concluded that the Atkins (extreme carbohydrate restriction of 20-50 g/day) and Zone (moderate carbohydrate restriction, 40% of total energy intake) diets led to greater weight loss and increased high-density lipoprotein (HDL) cholesterol levels more than the points-based Weight Watchers diet and the low-fat (10% of energy intake) vegetarian Ornish diet.14 Such studies also show that the efficacy of any diet is directly related to adherence. In a study by Alhassan et al., adherence scores were highest for the Atkins diet, but for all diets the greatest weight loss was seen in individuals with the highest adherence scores.15 It thus seems that diets can be successful so long as they are adequately adhered to. Another point to bear in mind is that the success of popular weight-loss diets is also dependent on the inclusion of a compulsory, rather than merely recommended, exercise program. For example, in another study comparing different weight-loss diets, the diet that included an exercise program led to the greatest improvements in triglyceride and insulin levels and the Homeostasis Model Assessment for Insulin Resistance (HOMA-IR) index, an indicator of insulin resistance, as well as weight loss.16
The above studies have various limitations. Their population samples are frequently small, with short follow-up periods, they provide little information on adherence, the interventions are of varying intensity, male subjects tend to be under-represented (hampering generalization of their results), the study design is usually not blinded, and the proportion of subjects lost to follow-up can be large (15-50% at one year). For these reasons, other forms of analysis are recommended.
Meta-analysesSeveral meta-analyses have been published in recent years aimed at overcoming some of the limitations of the small-scale studies described above. Hu et al. analyzed various randomized controlled clinical trials and demonstrated that low-carbohydrate and low-fat diets are equally effective in controlling weight, blood pressure and fasting blood glucose, but that low-carbohydrate diets resulted in lower reduction in total cholesterol and low-density lipoprotein cholesterol, but a greater increase in HDL cholesterol and a greater decrease in triglycerides.17 However, concerns have been raised about an increase in all-cause mortality (but not cardiovascular mortality or disease) associated with low-carbohydrate diets, as shown in a meta-analysis by Noto et al., who point out that these diets result in increased intake of protein from animal sources and of saturated fat and reduced intake of fruits, vegetables and fiber.18 A meta-analysis of studies on the Mediterranean diet with over 530 000 participants showed an overall beneficial effect of this diet on all risk factors, particularly triglyceride levels.19 A more recent meta-analysis also demonstrated the benefits of the Mediterranean diet in five of the six parameters assessed (only HDL cholesterol did not improve significantly).20 Steckhan et al. analyzed the effect of different dietary approaches on other parameters associated with MS, particularly inflammatory markers, in a meta-analysis that concluded that low-carbohydrate diets and multimodal interventions (those that combined diet with exercise and behavioral components) were most likely to lead to weight loss and decreased insulin levels.21 Among inflammatory parameters, only C-reactive protein was significantly affected, with the largest decrease resulting from low-fat diets.
Clinical trialsUnfortunately, meta-analyses are also not the ideal tool to examine the question of macronutrient composition for individuals with MS, since their results are limited by significant differences between studies. Randomized clinical trials, in turn, may also not be considered suitable for this purpose, since their participants tend to differ from real-world patients and they are carried out under tightly controlled conditions that are difficult to reproduce in the real world. Nevertheless, there have been two large clinical trials that are worth examining in detail.
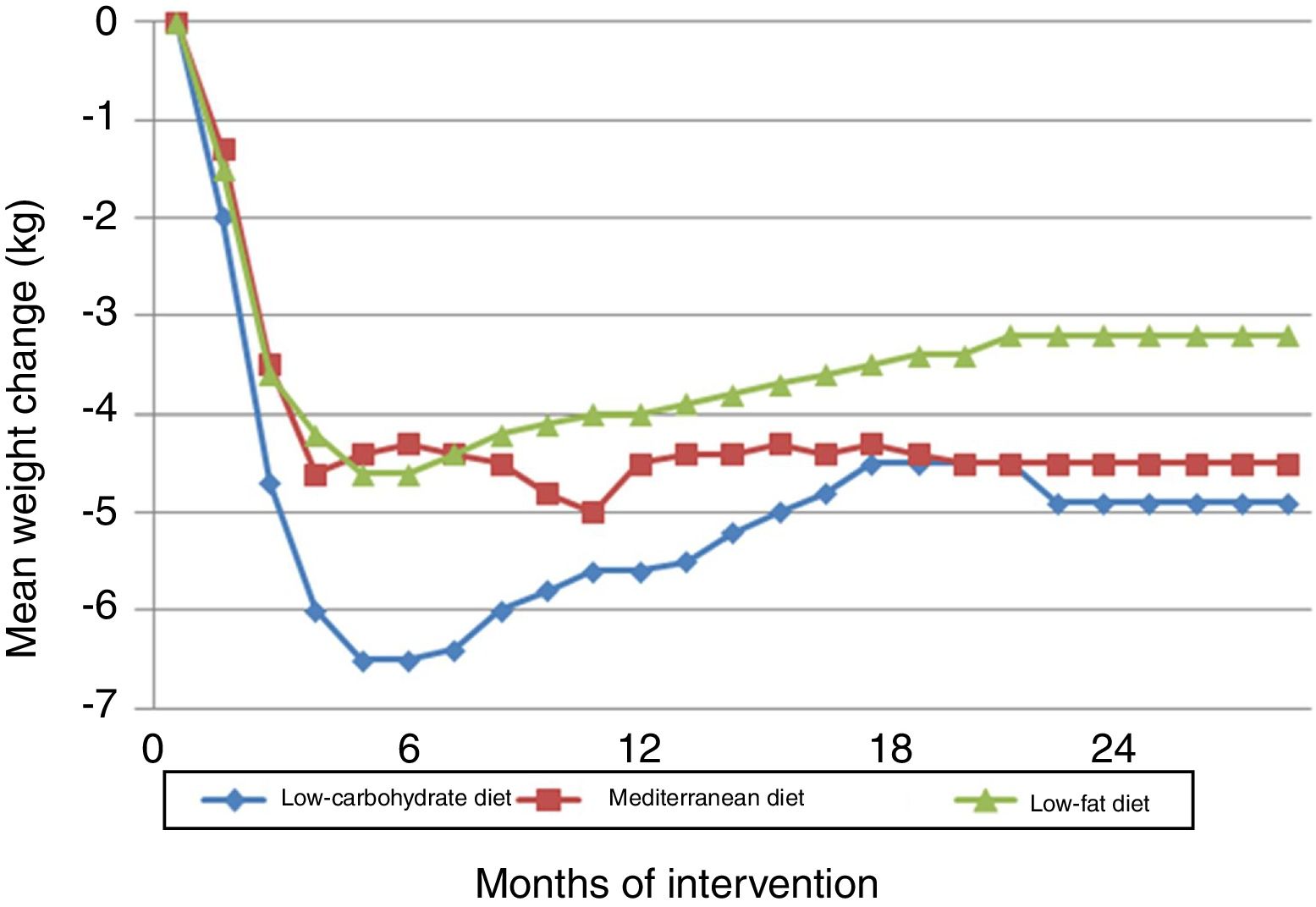
The Dietary Intervention Randomized Controlled Trial (DIRECT) compared three diets: low-fat, restricted-calorie (50% carbohydrate, 19% protein, 30% fat); low-carbohydrate, non-restricted-calorie (40% carbohydrates, 21% protein, 39% fat); and Mediterranean, restricted-calorie (50% carbohydrates, 19% protein, 33% fat).22 The trial took place in the workplace cafeteria of a medical clinic. It showed that all three diets, particularly the low-carbohydrate diet, were effective in terms of weight loss in the first six months, after which there was some weight gain, which stabilized from 12-18 months onward (Figure 1). HDL cholesterol and triglyceride levels improved most in the low-carbohydrate group, followed closely by the Mediterranean diet group. No significant differences were seen in other parameters including fasting blood glucose and insulin levels and the HOMA-IR index, except among diabetic subjects, in whom these parameters improved with the low-carbohydrate and Mediterranean diets. Overall adherence was excellent at 95.4% at 12 months and 84.6% at 24 months (90.4% for the low-fat diet, 85.3% for the Mediterranean diet and 78.0% for the low-carbohydrate diet).
Changes in weight in the Dietary Intervention Randomized Controlled Trial (DIRECT).22
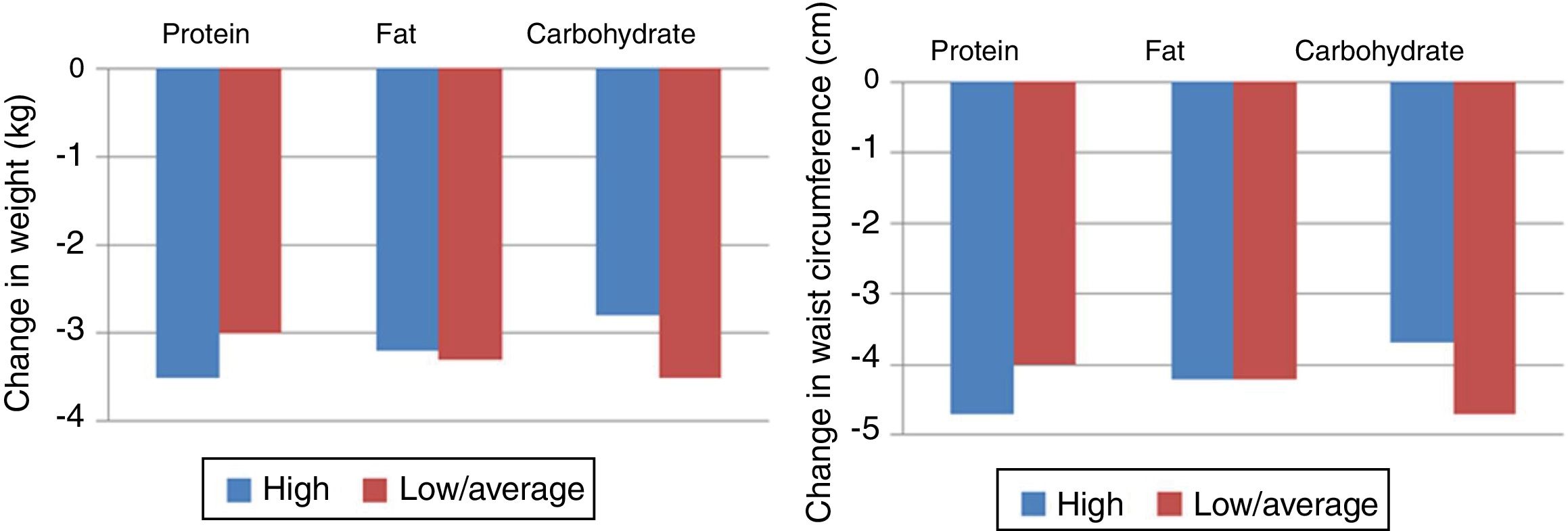
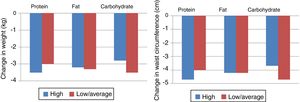
Another clinical trial, Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST), compared the macronutrient composition of different diets and concluded that weight loss was similar for all groups, with some weight gain at 12 months but with improvements in triglycerides, HDL cholesterol and fasting serum insulin in the low- or moderate-carbohydrate diets, although overall the prevalence of MS did not improve significantly (Figure 2).23 Difficulty in maintaining adequate adherence to the target levels for macronutrients was reported despite intensive behavioral counseling, and adherence was associated with level of weight loss. The lowest adherence rates were seen with the low-fat diet, which was the most different from the usual dietary patterns in the USA, where the trial took place.
Changes in weight and waist circumference in the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial.23
The question of the macronutrient composition of weight-loss diets recently resurfaced with the publication of the Prospective Urban Rural Epidemiology (PURE) study,24 a large epidemiological cohort study of 135 335 subjects with a long median follow-up of 7.4 years. Higher carbohydrate intake was associated with an increased risk of total and non-cardiovascular mortality, while higher total fat intake (saturated, unsaturated and polyunsaturated) was associated with lower risk of total mortality. No significant association was found between any of the diets and the occurrence of myocardial infarction or cardiovascular mortality. These surprising results may influence future international dietary guidelines.
The futureThere is extensive research on the ideal macronutrient composition for individuals with MS, including on less well-studied aspects of the syndrome. Genetic studies have demonstrated that the quantity and type of fats in foods affect regulation of lipid metabolism through the expression of genes involved in lipolysis and lipogenesis, suggesting a possible interaction between genetics and dietary interventions.25–27 The clinical trials referred to above provided a large sample of genetic data that on subsequent analysis show that some genetic variants of proteins involved in lipid metabolism or peripheral insulin signal transduction may respond differently to certain types of diet. For example, triglyceride and HDL cholesterol levels respond better to a high-fat diet in individuals with the CC genotype of the rs3764261 variant of the cholesteryl ester transfer protein gene.25 Similarly, the CC genotype of the rs2943641 variant of the insulin receptor substrate 1 gene and the TT genotype of the rs2287019 variant of the glucose-dependent insulinotropic polypeptide receptor are associated with improvements in weight loss, fasting insulin, fasting glucose and HOMA-IR index.26,27
Finally, research is under way into the role of gut microbiota.28 These symbiotic microorganisms are involved in the degradation of polysaccharides and oligosaccharides, promote intestinal impermeability, and are a source of vitamins B and K. Their composition is dependent on physiological and environmental factors. The gut flora of obese but metabolically healthy individuals (those without other metabolic risk factors and low visceral fat) is very different from that of metabolically unhealthy obese subjects, which suggests that gut microbiota play an important role. It has been demonstrated that dietary interventions using prebiotic or bioactive nutrients may help switch a metabolically unhealthy obese state to a metabolically healthy one. In particular, intake of foods rich in medium chain free fatty acids and medium chain triglycerides (which are hydrolyzed faster than long chain triglycerides, are capable of passive diffusion across cell membranes, and are transported directly rather than in chylomicrons following intestinal absorption) can modify gut microbiota, improving intestinal impermeability to macrophages and reducing associated inflammation.
ConclusionAll the diets described above may lead to weight loss, particularly in the first six months; the loss is slightly greater with low-carbohydrate diets. After this initial success, some weight gain is usually seen from 12 months onward, followed by stabilization up to two years. With regard to metabolic risk factors, HDL cholesterol and triglycerides appear to respond more favorably to a low-carbohydrate or Mediterranean diet, as do parameters of insulin resistance. Blood pressure and other parameters, including adiponectin and leptin levels, are not significantly affected by the type of diet. The greatest benefits are obtained in individuals who maintain strict long-term adherence to their diet.
On the basis of these findings, it is reasonable to argue that rather than choosing a particular macronutrient composition, it may be more valuable to analyze each individual's usual dietary pattern and identify a diet that he or she can follow in the long term. This allows consideration of personal preferences when drawing up the details of the dietary plan. Success is more likely with a diet that includes moderate quantities of macronutrients, so long as a moderate energy intake is maintained and it is associated with an exercise program; when necessary other interventions, including behavioral components, should be available.
In the future, further information may become available on how to adjust diets to an individual's genetic characteristics or gut flora, but the above principles should always be borne in mind.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Timóteo AT. Dieta em doentes com síndrome metabólica: qual a composição ideal de macronutrientes?. Rev Port Cardiol. 2018;37:1001–1006.