Exercise with flexible poles provides fast eccentric and concentric muscle contractions. Although the literature reports significant muscle chain activity during this exercise, it is not clear if a single bout of exercise induces cardiac changes. In this study we assessed the acute effects of flexible pole exercise on cardiac autonomic regulation.
MethodsThe study was performed on 22 women between 18 and 26 years old. We assessed heart rate variability (HRV) in the time (SDNN, RMSSD and pNN50) and frequency (HF, LF and LF/HF ratio) domains and geometric indices of HRV (RRTri, TINN, SD1, SD2 and SD1/SD2 ratio). The subjects remained at rest for 10 min and then performed the exercises with the flexible poles. Immediately after the exercise protocol, the volunteers remained seated at rest for 60 min and HRV was analyzed.
ResultsWe observed no significant changes in time domain (SDNN: p=0.72; RMSSD: p=0.94 and pNN50: p=0.92) or frequency domain indices (LF [nu]: p=0.98; LF [ms2]: p=0.72; HF [nu]: p=0.98; HF [ms2]: p=0.82 and LF/HF ratio: p=0.7) or in geometric indices (RRTri: p=0.54; TINN: p=0.77; SD1: p=0.94; SD2: p=0.67 and SD/SD2: p=0.42) before and after a single bout of flexible pole exercise.
ConclusionA single bout of flexible pole exercise did not induce significant changes in cardiac autonomic regulation in healthy women.
Exercícios com hastes flexíveis proporcionam rápidas contrações musculares excêntricas e concêntricas. Embora a literatura relate importante ativação da cadeia muscular durante este exercício, não é claro se uma única sessão de exercício induz alterações cardíacas. Neste estudo foram avaliados os efeitos agudos da haste flexível sobre a regulação autonômica cardíaca.
MétodosO estudo foi realizado em 22 mulheres entre 18 e 26 anos. Avaliou-se a variabilidade da freqüência cardíaca (VFC) no domínio do tempo (SDNN, RMSSD e pNN50) e no domínio da frequência (HF, LF e LF relação/HF) e os índices geométricos de VFC (RRtri, TINN, SD1, SD2 e a razão SD1/SD2). Os indivíduos permaneceram em repouso por 10 minutos. Após o período de repouso, os voluntários realizaram os exercícios com as hastes flexíveis. Imediatamente após o protocolo de exercício, os voluntários permaneceram sentados em repouso por 60 minutos e a VFC foi analisada.
ResultadosNão foram observadas alterações no domínio do tempo (SDNN: p=0,72; RMSSD: p=0,94 e pNN50: p=0,92) e nos índices no domínio da frequência (LF (nu): p=0,98; LF (ms2): p=0,72; HF (nu): p=0,98; HF (ms2): p=0,82 e a razão LF/HF: p=0,7), bem como para os índices geométricos (RRtri: p=0,54; TINN: p=0,77; SD1: p=0,94; SD2: p=0,67 e SD/SD2: p=0,42) entre o antes e o depois de um único exercício com haste flexível.
ConclusãoUma única sessão de exercício com vara flexível não induziu mudanças na regulação autonômica cardíaca em mulheres saudáveis.
The autonomic nervous system plays a major role in the modulation of the cardiovascular system in various situations,1 including exercise.2 In order to maintain cardiovascular homeostasis during exercise, mechanisms based on the rapid action of the autonomic nervous system on the heart are necessary.2 The cardiovascular responses induced by exercise are characterized by immediate parasympathetic withdrawal at the beginning of exercise, increasing heart rate, followed by an increase in sympathetic nervous system activity. Immediately after exercise, heart rate falls due to vagal reactivation.3 Cardiac autonomic responses after exercise are important indicators of risk for cardiac events.4
In this context, heart rate variability (HRV) is a non-invasive method that assesses cardiac autonomic regulation by analyzing variations in the intervals between consecutive heart beats (RR intervals) that are related to the influence of the autonomic nervous system on the sinus node.5 At rest, high HRV is an indicator of good adaptation, as in athletes, while reduced HRV may indicate cardiac impairment.6,7 During endurance exercise HRV is modulated by reduced vagal tone and increased sympathetic modulation, while after the end of exercise HRV progressively increases.8
Studies of cardiac autonomic response after resistance exercise show less intense responses compared to endurance exercise.9 Isometric contractions with different intensities have also been observed to change vagal modulation of the heart.10 Considering that endurance exercise induces greater cardiac overload, this style of exercise will induce a stronger cardiac autonomic response in the recovery phase compared to resistance exercise.9
The flexible pole is an instrument that produces muscle contractions generated by co-contraction of the shoulder and trunk muscles.10,11 Although flexible pole exercise has been used in rehabilitation therapy for shoulder instability,12 there is little research in the literature on its acute effects on cardiac autonomic regulation as a part of cardiovascular rehabilitation programs. Furthermore, additional modalities for cardiac therapy are always welcome. This study was therefore undertaken to assess the acute effects of a standardized exercise protocol with flexible poles on cardiac autonomic regulation.
MethodsStudy populationThe study was performed on 22 healthy female subjects, all nonsmokers, aged between 18 and 26 years. All volunteers were informed about the procedures and objectives of the study and gave their written informed consent. All study procedures were approved by the Research Ethics Committee of the Faculty of Science of Universidade Estadual Paulista, Marilia Campus (study no. 0554-2012), and were in accordance with National Health Council Resolution no. 196/96 of 10/10/1996.
Exclusion criteriaSubjects were excluded under the following circumstances: body mass index (BMI) >35 kg/m2; systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg at rest; cardiac arrhythmias (atrial flutter or fibrillation, multiple ventricular or atrial ectopy, second- or third-degree atrioventricular block), smoking, left ventricular dysfunction, neurological or respiratory disorders, severe postural deviation such as severe scoliosis, kyphosis or hyperboloids that could influence respiratory pattern, and auditory disorders. In order to avoid effects related to sex hormones we did not include women on the 11th–15th and 21th–25th days after the first day of their menstrual cycle.13 Physically active subjects according to the International Physical Activity Questionnaire14 were not included.
Initial assessmentPrior to the study, the following baseline parameters were recorded: age, gender, weight, height and BMI. Weight was determined using a digital scale (W200/5, Welmy, Brazil) with a precision of 0.1 kg. Height was determined using a stadiometer (ES2020, Sanny, Brazil) with a precision of 0.1 cm. BMI was calculated as weight in kg divided by height in m squared.
Heart rate variability analysisRR intervals recorded by the portable RS800CX heart rate monitor (Polar Electro®, Finland) with a sampling rate of 1000 Hz were downloaded to Polar Precision Performance software, version 3.0 (Polar Electro, Finland). The software enables visualization of heart rate and extraction of a text file recording RR intervals. After digital filtering supplemented with manual filtering to eliminate artefacts and premature ectopic beats, a minimum of 256 RR intervals were used for data analysis. Only series with more than 95% of sinus beats were included in the study.5 For calculation of linear indices we used the HRV analysis software Kubios HRV version 1.1 for Windows (Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Finland).15
Linear indices of heart rate variabilityTo analyze HRV in the frequency domain, low-frequency (LF; 0.04–0.15 Hz) and high-frequency (HF; 0.15–0.40 Hz) spectral components were recorded in ms2 and normalized units (nu), representing a value relative to each spectral component in relation to the total power minus the very-low-frequency (VLF) components, and the ratio between these components (LF/HF). The spectral analysis was performed using the fast Fourier transform algorithm.6
Analysis in the time domain was performed in terms of SDNN (standard deviation of normal-to-normal RR intervals), pNN50 (percentage of adjacent RR intervals differing by more than 50 ms) and RMSSD (root mean square of successive differences between adjacent normal RR intervals).6
Experimental protocolData collection was carried out in the same soundproof room for all volunteers at a temperature between 21°C and 25°C and relative humidity between 50 and 60%. Volunteers were instructed not to drink alcohol or caffeine for 24 hours before assessment and to eat no more than a light meal in the two hours before experiments. Data were collected on an individual basis, between 6 and 9 pm, to standardize the protocol. All procedures necessary for data collection were explained on an individual basis and the subjects were instructed to remain at rest and to avoid talking during data collection.
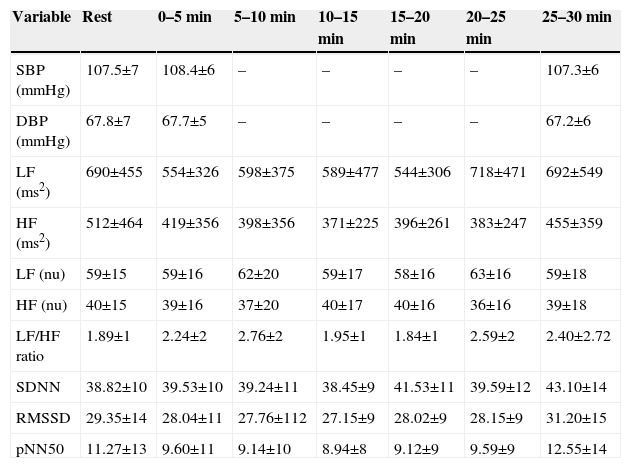
After the initial assessment the heart monitor belt was placed over the thorax and aligned with the distal third of the sternum, and the Polar RS800CX heart rate receiver was placed on the wrist. Before starting the exercises, the volunteers received visual feedback through a monitor to maintain a neutral standing posture and were instructed to maintain the same posture throughout the exercise.16 SBP and DBP were measured before and immediately after exercise and 30 min later. The oscillatory movement of the flexible pole (Flexibar®) was maintained by elbow flexion and extension. The flexible pole vibrated at a frequency of 5 Hz, this frequency being based on an auditory stimulus from a metronome (Quartz Metronome®) calibrated at 300 bpm.10
The flexible pole exercises were conducted with volunteers in a standing position with feet apart (wide base) and shoulder flexion as the proposed position. To maintain the proper flexion in each shoulder visual feedback was used as a target. All exercises were performed for 15 s with 50–60 s of rest between the exercises. Three repetitions were performed for each exercise.10
The exercises were performed in the following positions: (1) with shoulders at approximately 180° flexion with the pole in the frontal plane, parallel to the ground (Figure 1A); (2) with the shoulders at 90° flexion with the pole in the transverse plane (Figure 1B); and (3) with one shoulder at 90° flexion with the pole in the sagittal plane, perpendicular to the ground (Figure 1C). HRV was analyzed during the following periods: control (resting), 0–5 min, 5–10 min, and 10–15 min, 15–20 min, 20–25 min and 25–30 min after the exercise protocol.
The three positions of the flexible pole exercise protocol: with shoulders at approximately 180° flexion with the pole in the frontal plane, parallel to the ground (A); with the shoulders at 90° flexion with the pole in the transverse plane (B); and one shoulder at 90° flexion with the pole in the sagittal plane, perpendicular to the ground (C).
Standard statistical methods were used to calculate means and standard deviations. The normal Gaussian distribution of the data was verified by the Shapiro-Wilk goodness-of-fit test (z value >1.0). For parametric distributions, one-way ANOVA was applied for repeated measures followed by the Bonferroni post-test. For nonparametric distributions, the Friedman test was used followed by Dunn's post-test. Differences were considered significant when the probability of a type I error was less than 5% (p<0.05). Biostat 2009 Professional 5.8.4 software was used for the statistical analysis.
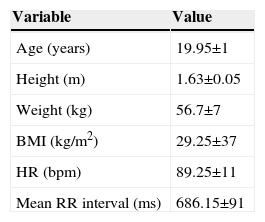
ResultsTable 1 presents data on baseline heart rate and mean RR interval, age, height, body weight and BMI.
Table 2 shows the behavior of SBP and DBP and time and frequency domain indices of HRV before and after a single bout of flexible pole exercise. The time domain indices SDNN, RMSSD and pNN50 did not change after exercise and the frequency domain indices LF (nu and ms2), HF (nu and ms2) and LF/HF ratio did not exhibit significant responses induced by a single bout of flexible pole exercise.
Diastolic and systolic blood pressure before, and time and frequency domain indices before and after a standardized flexible pole exercise protocol.
| Variable | Rest | 0–5 min | 5–10 min | 10–15 min | 15–20 min | 20–25 min | 25–30 min |
|---|---|---|---|---|---|---|---|
| SBP (mmHg) | 107.5±7 | 108.4±6 | – | – | – | – | 107.3±6 |
| DBP (mmHg) | 67.8±7 | 67.7±5 | – | – | – | – | 67.2±6 |
| LF (ms2) | 690±455 | 554±326 | 598±375 | 589±477 | 544±306 | 718±471 | 692±549 |
| HF (ms2) | 512±464 | 419±356 | 398±356 | 371±225 | 396±261 | 383±247 | 455±359 |
| LF (nu) | 59±15 | 59±16 | 62±20 | 59±17 | 58±16 | 63±16 | 59±18 |
| HF (nu) | 40±15 | 39±16 | 37±20 | 40±17 | 40±16 | 36±16 | 39±18 |
| LF/HF ratio | 1.89±1 | 2.24±2 | 2.76±2 | 1.95±1 | 1.84±1 | 2.59±2 | 2.40±2.72 |
| SDNN | 38.82±10 | 39.53±10 | 39.24±11 | 38.45±9 | 41.53±11 | 39.59±12 | 43.10±14 |
| RMSSD | 29.35±14 | 28.04±11 | 27.76±112 | 27.15±9 | 28.02±9 | 28.15±9 | 31.20±15 |
| pNN50 | 11.27±13 | 9.60±11 | 9.14±10 | 8.94±8 | 9.12±9 | 9.59±9 | 12.55±14 |
DBP: diastolic blood pressure; HF: high frequency; LF: low frequency; nu: normalized units; pNN50: percentage of adjacent RR intervals differing by more than 50 ms; RMSSD: root mean square of successive differences between adjacent normal RR intervals; SBP: systolic blood pressure; SDNN: standard deviation of normal-to-normal RR intervals. Values are mean ± standard deviation.
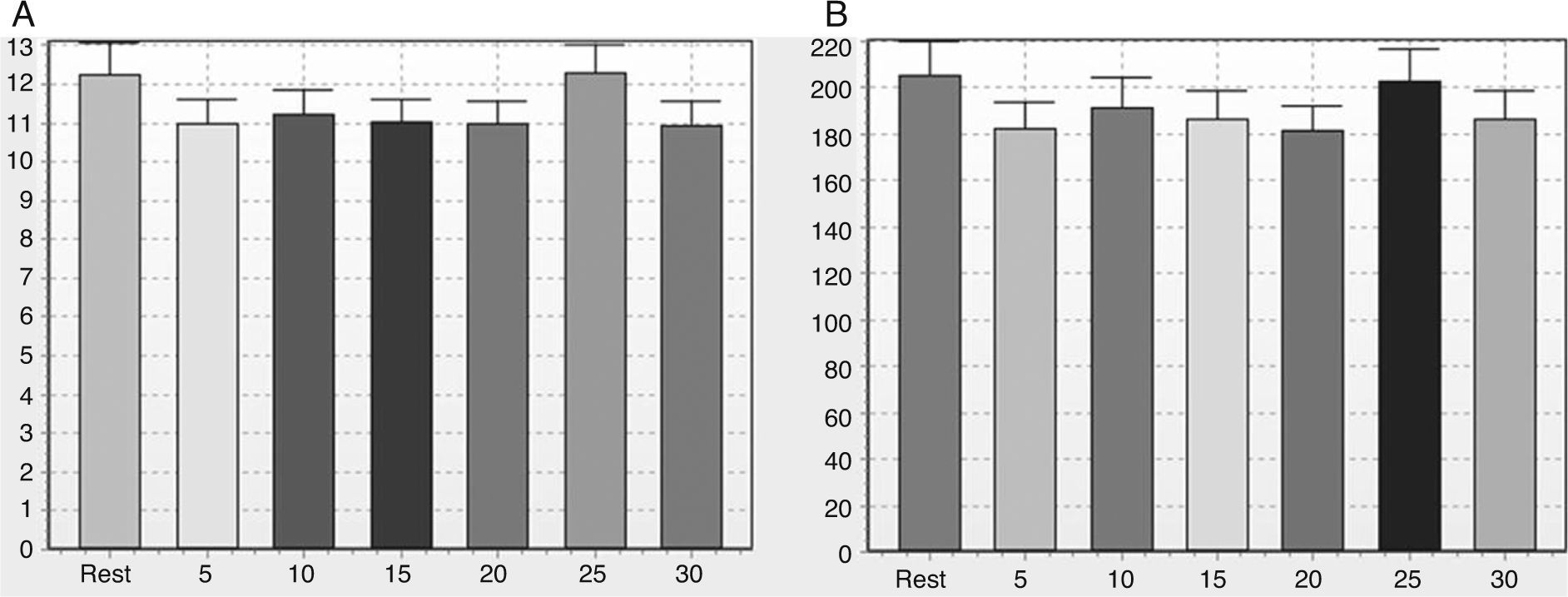
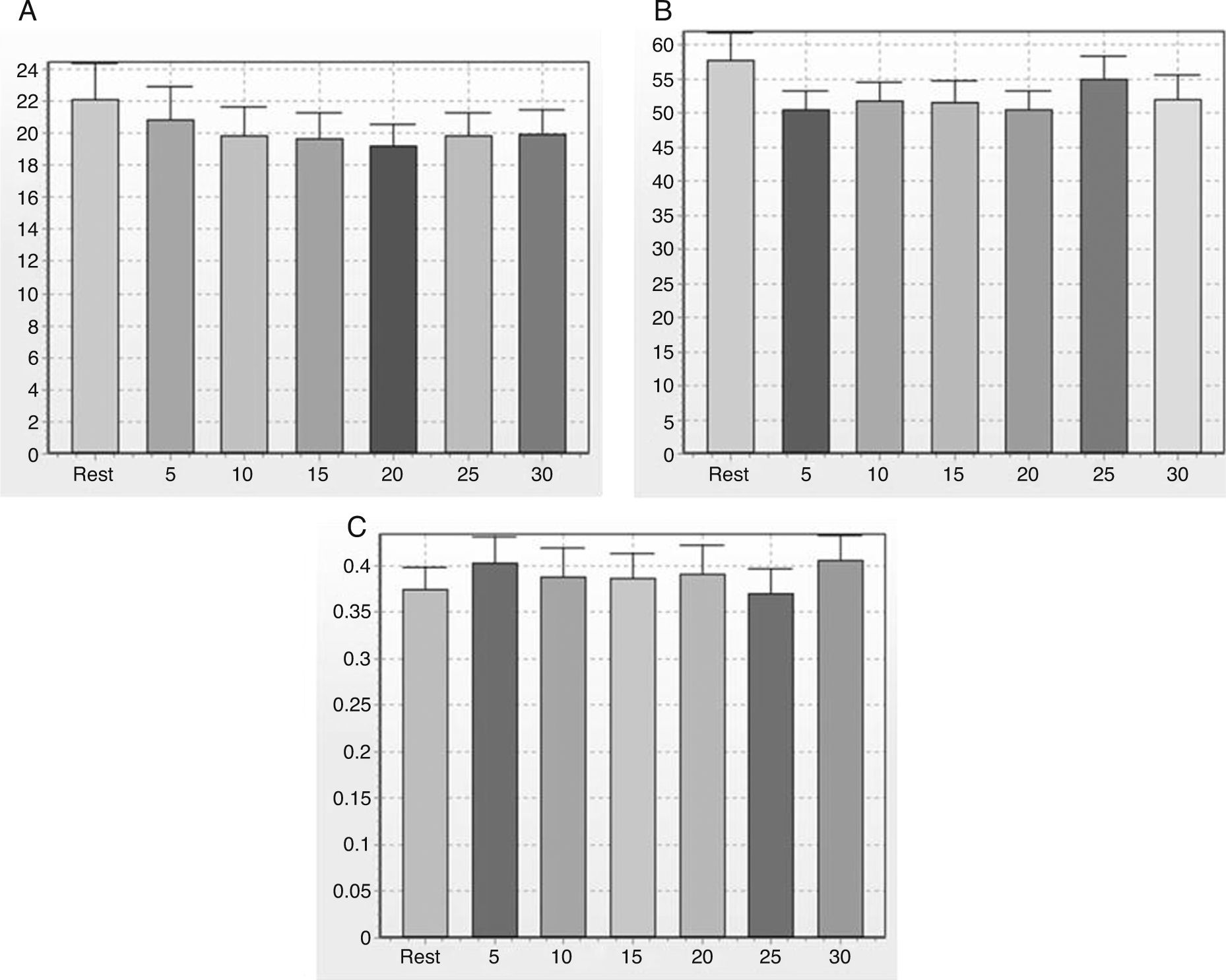
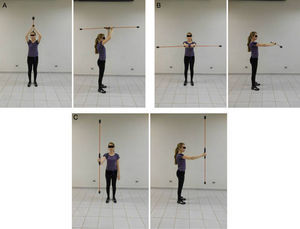
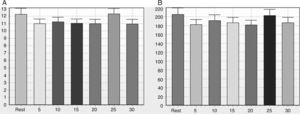
Note in Figure 2 that the geometric indices of HRV (triangular index of RR intervals, RRTri, and triangular interpolation of RR intervals, TINN) did not change significantly after one session of flexible pole exercise. Moreover, the Poincaré plot indices – SD1 (standard deviation of instantaneous beat-to-beat variability), SD2 (standard deviation of long-term continuous RR interval variability), and SD1/SD2 ratio – did not present significant differences before and after the exercise protocol (Figure 3).
SD1 (A), SD2 (B) and SD1/SD2 (C) ratio before and after a standardized exercise protocol with flexible pole. SD1: standard deviation of instantaneous beat-to-beat variability; SD2: standard deviation of long-term continuous RR interval variability; SD1/SD2 ratio: ratio between short- and long-term RR interval variability. Times in min.
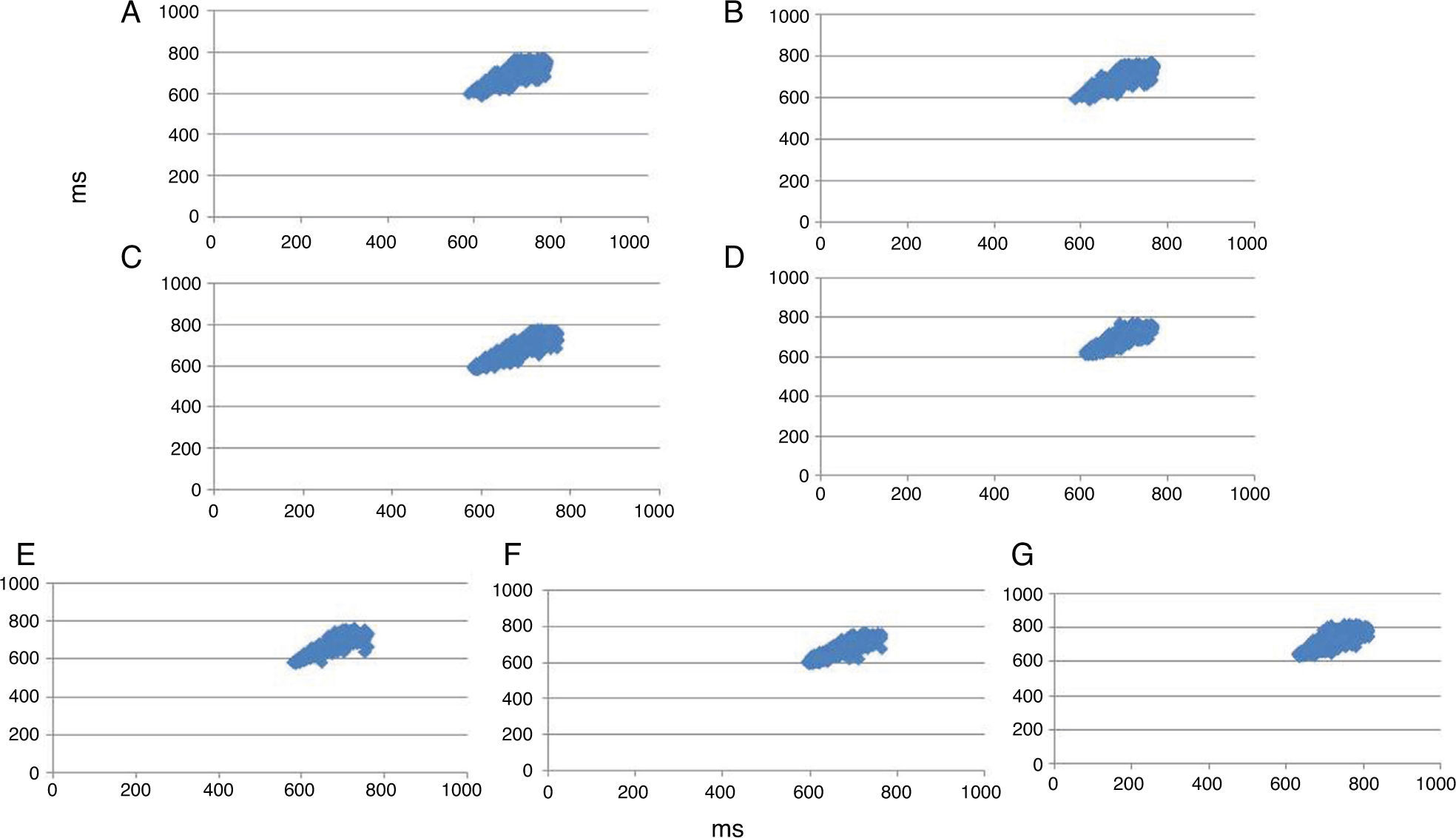
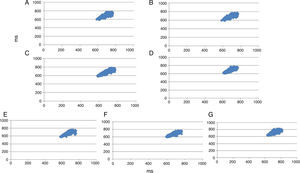
Figure 4 shows an example of the Poincaré plot patterns from one subject in the control state before exercise (A), 0–5 min (B), 5–10 min (C), 10–15 min (D), 15–20 min (E), 20–25 min (F) and 25–30 min (G) after a standardized flexible pole exercise protocol.
DiscussionIsometric muscle activation acutely elicits cardiovascular responses through mechanoreceptors.17 Isometric leg extension and isometric handgrip are reported to induce increases in skin sympathetic nerve activity, which is thus a potential measure for analysis of autonomic nervous system during muscle contraction.18 Flexible pole exercise activates isometric contraction of muscles that affect intrathoracic pressure, such as the internal oblique muscle,10 and also muscles related to shoulder movements, including the upper trapeziums, lower trapeziums and lateral deltoids muscles.11 However, there are few studies in the literature on the acute effects of flexible pole exercise on cardiovascular variables. In this study we assessed cardiac autonomic recovery after a single session of standardized flexible pole exercise in healthy women. The main result was absence of significant responses of HRV through analysis of time and frequency domain and geometric indices after exercise.
According to our data, there were no significant differences in SBP and DBP between before, immediately after and 30 min after the exercise protocol. Cardiovascular recovery after high-intensity aerobic exercise is characterized by decreased blood pressure (BP) after cessation of exercise compared to before exercise onset (post-exercise hypotension).19 However, flexible pole exercise is isometric and anaerobic, inducing contraction of trunk and shoulder muscles.10,11 It has been observed that BP increases in young men during static exercise at 40% of maximal voluntary static contraction of lower limb muscles.20 This result is supported by the literature, since activation of the muscle chemoreflex during sustained isometric contractions raises BP through increases in muscle sympathetic nerve activity.21 We believe that the intensity of the exercise protocol used in our study, rather than the style of exercise, was the main reason for the absence of significant responses of SBP and DBP during the recovery phase.
Three mechanisms have been proposed to explain cardiac autonomic control during voluntary muscle contraction: activation of brain areas (“central command”), based on the influence of forebrain areas such as the bed nucleus of the stria terminalis that regulates tachycardic responses induced by exercise in rats22; reflex activity, involving chemo- and mechanoreceptors; and baroreceptor afferents, associated with attenuation of baroreceptor function during exercise, which causes BP to rise without stimulating the bradycardic reflex.23 We initially hypothesized that this standardized exercise protocol would induce significant cardiac autonomic responses during the recovery phase, mainly due to the exercise pressor reflex. This is a peripheral neural reflex originating in skeletal muscle that contributes to the regulation of the cardiovascular system during exercise. The afferent arm of this reflex is composed of mechanically sensitive (mostly group III) and metabolically sensitive (mainly group IV) fibers.24 In the recovery phase of exercise, mechanoreceptors were assumed to present stronger acute effects on HRV elicited by flexible pole exercise, since each exercise lasted 15 s, which is not long enough to cause significant changes in metabolites and thus to lead to metaboreflex activation. On the other hand, no change was reported before and after the standardized protocol in our study.
In our study, exercises were performed in three positions: (1) with shoulders at approximately 180° flexion with the pole in the frontal plane, parallel to the ground; (2) with the shoulders at 90° flexion with the pole in the transverse plane; and (3) with one shoulder at 90° flexion with the pole in the sagittal plane, perpendicular to the ground. The standardized protocol was based on exercises performed for 15 s with 50–60 s of rest between each exercise and three repetitions for each exercise, in accordance with the literature.10 It is possible that if the exercise duration were 30 s instead of 15 s the cardiac autonomic responses would be more intense. Nonetheless, we did not use a protocol based on moderate to high intensity exercise, since we wished to develop a rehabilitation program that would be useful to patients with cardiac disorders.
The intensity of flexible pole exercise used in this study was based on previous research,10,11 which proposed the oscillation frequency of the flexible pole according to auditory stimulation from a metronome set to 300 bpm. Intensity of flexible pole exercise can be controlled by regulating the rate of the auditory stimulation, i.e. lower intensity for fewer bpm on the metronome.
Flexible pole exercise leads to isometric activation of shoulder and trunk muscles.10,11 Autonomic and cardiac variables have been analyzed during isometric leg extension exercises and isometric handgrip.18 The authors reported that the sympathetic activation induced by exercise depends on its intensity and that the magnitude of exercise is not associated with the number of muscle fibers involved or with the exercising limb. We therefore suggest that increasing the frequency of auditory stimulation could enhance cardiac autonomic recovery response.
Besides analysis of the linear time and frequency domain indices of HRV, we investigated the non-linear indices of HRV through Poincaré plot indices (SD1, SD2 and SD1/SD2 ratio). The SD1 index measures the dispersion of points perpendicular to the line of identity; it reflects short-term RR variability and is an indicator of vagal cardiac modulation. The SD2 index corresponds to the dispersion of the points along the line of identity; it indicates long-term HRV and is an indicator of sympathetic and parasympathetic cardiac modulation.25 The Poincaré plot describes the nonlinear dynamics of a phenomenon; it is a visual technique to identify the hidden correlation patterns of a time series signal.26 Nonlinear analysis mechanisms are thought to be involved in the genesis of heart rate dynamics27 and nonlinear analysis seems to be more sensitive to HRV changes compared to linear analysis.7 Even though the indices that are more sensitive did not change significantly between before and after flexible pole exercise, further studies are required to investigate the safety of this standardized exercise protocol in patients with cardiovascular disease.
A major finding of our study is that a single bout of flexible pole exercise caused no significant changes in the time domain, frequency domain or geometric indices of HRV compared to the resting control state in healthy women. The literature shows that greater sympathetic increase and parasympathetic reduction after exercise are significant predictors of critical and non-fatal cardiovascular events.4 A study with low-intensity resistance exercise in patients with peripheral artery disease reported no significant change in HRV.28 We suggest that additional studies should be performed in patients with cardiovascular disorders.
Flexible pole exercise has been used for rehabilitation programs to treat shoulder instability.29 However, there is little research in the literature on its use for cardiovascular and respiratory therapy. It is important to know whether low to moderate exercise protocols are more indicated for patients with cardiovascular impairment in the initial phase of the rehabilitation,30 because attenuated parasympathetic tone can lead to sudden death.4
Physical exercise has positive acute and chronic effects on cardiac patients, such as decreasing BP, which justifies prescribing it in association with drug therapy to achieve rapid and lasting improvements in their general medical condition.31 The best type of exercise to reduce morbidity and mortality in cardiovascular patients has long been sought. In this context, we set out to develop an exercise protocol that can be indicated for such patients.
Our study presents some limitations. We investigated only women, because there is evidence indicating significant differences between men and women regarding cardiac autonomic regulation during the recovery phase of exercise.32 Moreover, considering that the menstrual cycle has been reported to influence baseline nonlinear HRV properties,13 we did not include volunteers during the follicular and luteal phases of the menstrual cycle. Other limitations include the absence of electromyographic analysis and gas analysis; however, our focus was on cardiac autonomic response after exercise.
ConclusionA standardized flexible pole exercise protocol did not cause significant changes in cardiac autonomic regulation in healthy adult women. Further studies should be carried out to assess the long-term effects of this exercise protocol on cardiovascular variables.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study received financial support from FUNDUNESP and PROPe/UNESP.